- Original
- Open Access
- Published:
(1 → 3)-β-D-Glucan-guided antifungal therapy in adults with sepsis: the CandiSep randomized clinical trial
Frank Bloos, Jürgen Held, Stefan Kluge, et al
Intensive Care Med (2022). https://doi.org/10.1007/s00134-022-06733-x
Abstract
Purpose
To investigate whether (1 → 3)-β-d-Glucan (BDG)-guidance shortens time to antifungal therapy and thereby reduces mortality of sepsis patients with high risk of invasive Candida infection (ICI).
Methods
Multicenter, randomized, controlled trial carried out between September 2016 and September 2019 in 18 intensive care units enrolling adult sepsis patients at high risk for ICI. Patients in the control group received targeted antifungal therapy driven by culture results. In addition to targeted therapy, patients in the BDG group received antifungals if at least one of two consecutive BDG samples taken during the first two study days was ≥ 80 pg/mL. Empirical antifungal therapy was discouraged in both groups. The primary endpoint was 28-day-mortality.
Results
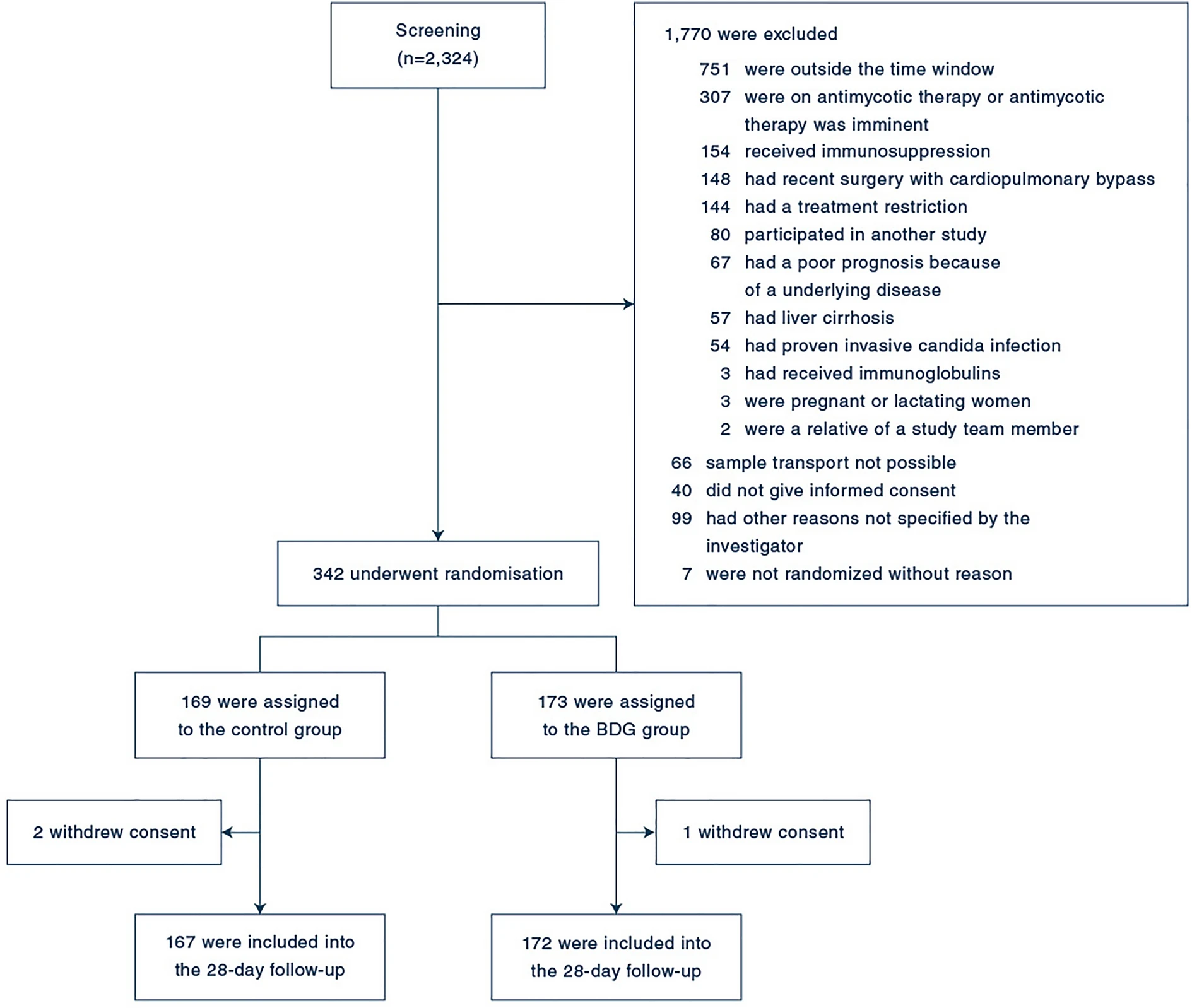
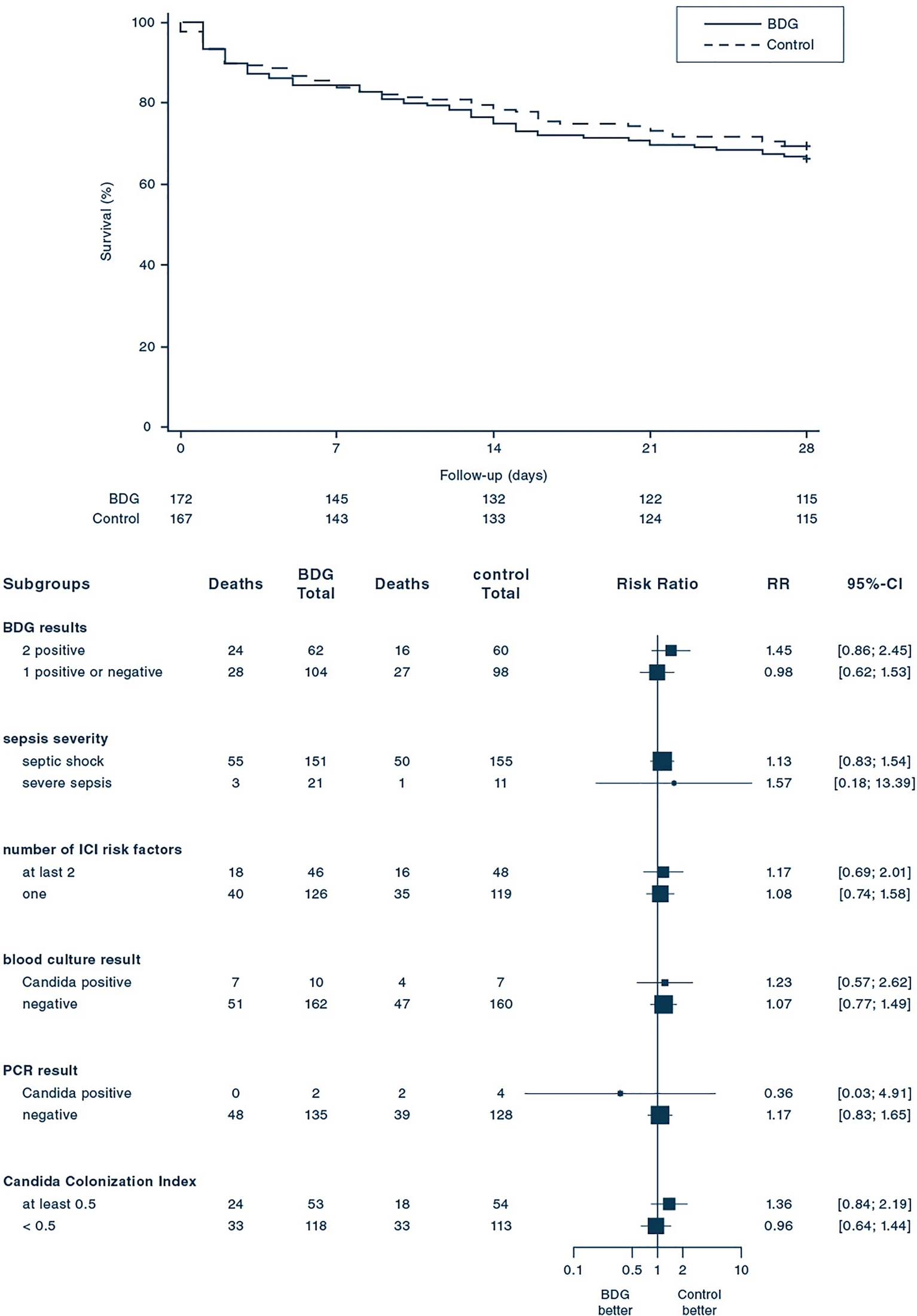
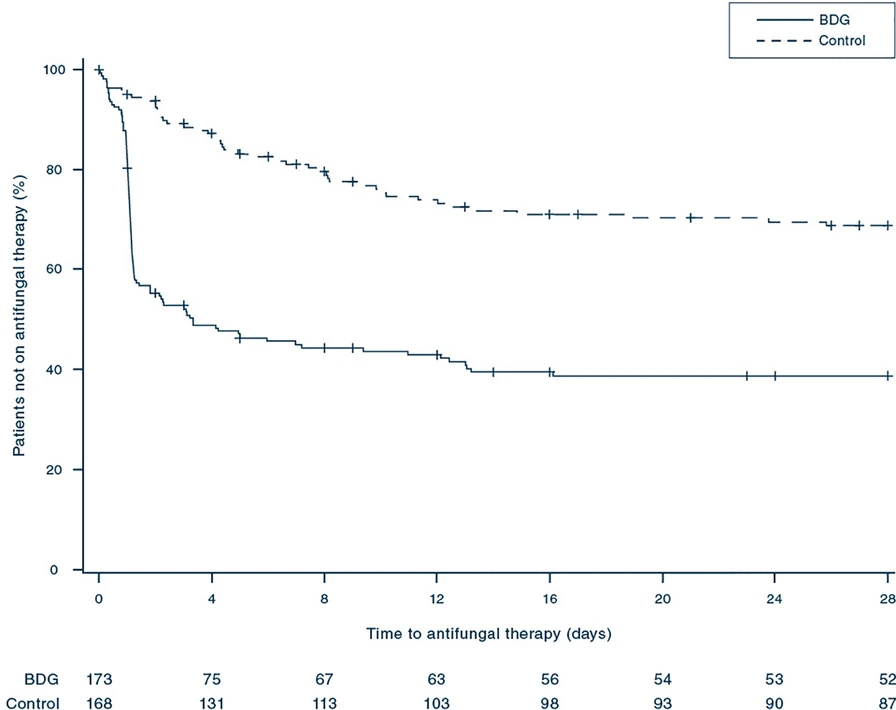
339 patients were enrolled. ICI was diagnosed in 48 patients (14.2%) within the first 96 h after enrollment. In the BDG-group, 48.8% (84/172) patients received antifungals during the first 96 h after enrollment and 6% (10/167) patients in the control group. Death until day 28 occurred in 58 of 172 patients (33.7%) in the BDG group and 51 of 167 patients (30.5%) in the control group (relative risk 1.10; 95% confidence interval, 0.80–1.51; p = 0.53). Median time to antifungal therapy was 1.1 [interquartile range (IQR) 1.0–2.2] days in the BDG group and 4.4 (IQR 2.0–9.1, p < 0.01) days in the control group.
Conclusions
Serum BDG guided antifungal treatment did not improve 28-day mortality among sepsis patients with risk factors for but unexpected low rate of IC. This study cannot comment on the potential benefit of BDG-guidance in a more selected at-risk population.