Anti-Inflammatory Strategies for Sepsis-Induced Lung Injury
- In ICU
- Tue, 20 May 2025
ICU Management & Practice, Volume 25 - Issue 2, 2025
Sepsis-induced ARDS is a critical condition that may require anti-inflammatory strategies. There are some promising treatments that may alter the evolution, including corticosteroids, vitamin C, NET inhibitors, and statins, which help mitigate excessive immune response and improve clinical outcomes.
Introduction
Sepsis represents a heterogeneous life-threatening condition triggered by acute infection and characterised by dysregulated host inflammatory response and multiple organ dysfunction. In a worldwide multicentre epidemiological survey, Rudd et al. (2020) showed an estimated 11 million total sepsis-related deaths worldwide in 2017 which accounted for almost 20% of global deaths that year. On the other hand, Acute Respiratory Distress Syndrome (ARDS) is an aggressive lung diffuse injury with rapid onset of widespread inflammation in the lungs and characterised by acute hypoxic respiratory failure associated with increased pulmonary vascular permeability, increased lung weight and loss of aerated lung tissue (Wang et al. 2024). In an observational prospective study carried out in 459 intensive care units (ICUs) in 50 countries of five continents (LUNG SAFE study), sepsis is the most common independent risk factor of ARDS development (Bellani et al. 2016). Thereby, ARDS is a key complication among patients with sepsis that can lead to significant morbidity and mortality. Indeed, in some recent data the proportion of non-surviving patients in sepsis-ARDS comorbidity reached 71% (Zhou et al. 2014).
Anti-Inflammatory Strategies for Sepsis-Induced Lung Injury
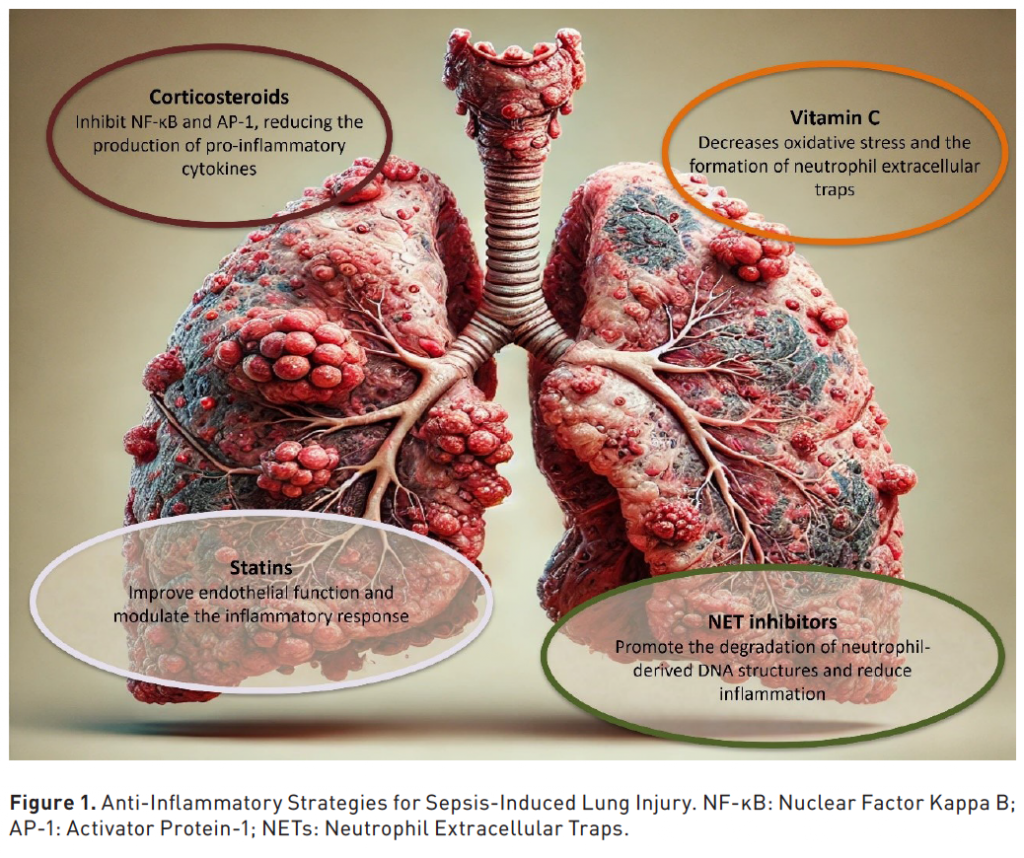
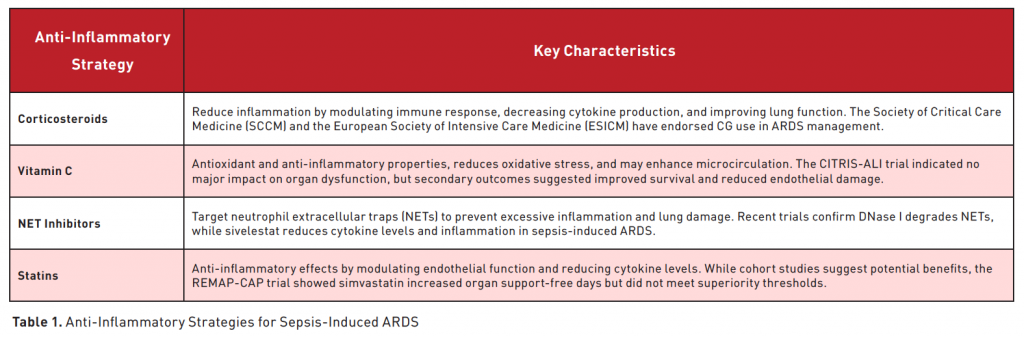
As previously mentioned, sepsis-induced ARDS remains a significant challenge in critical care, with high morbidity and mortality rates. Currently, treatment primarily involves supportive care, as no definitive pharmacological solution has been established. However, recent research indicates that anti-inflammatory agents such as corticosteroids and vitamin C may help reduce the excessive immune response driving the pathophysiology of ARDS. We will review medications and innovative therapies designed to address and mitigate the excessive immune responses associated with sepsis-induced ARDS (Figure 1) (Table 1).
1. Corticosteroids
In recent decades, corticosteroids (CGs) have been extensively studied for their role in managing inflammatory conditions. They play a crucial role in physiological processes such as metabolism, electrolyte balance, immune modulation, and inflammation regulation (Cruz-Topete and Dilowski 2015; Oakley and Cidlowski 2013). Under normal conditions, CGs are produced at low levels; however, during stress events like infections or trauma, the hypothalamic-pituitary-adrenal (HPA) axis is activated, leading to increased CG secretion to maintain homeostasis. CGs exert their effects through nuclear glucocorticoid receptors (GRs), which modulate gene expression, suppress inflammation, and alleviate oxidative stress (Zhao et al. 2018). Their primary anti-inflammatory actions involve reducing pro-inflammatory cytokine production, downregulating chemokines, and inhibiting nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) transcription factors (Zhang et al. 2023). These properties have positioned GCs as key agents in the treatment of acute respiratory distress syndrome (ARDS).
Preclinical studies have demonstrated the efficacy of CGs in alleviating lung injury. Yuan et al. (2024) found that methylprednisolone (MP) significantly reduced tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) levels in an LPS-induced acute lung injury (ALI) model, promoting alveolar epithelial cell survival. Kosutova et al. (2016) observed that dexamethasone (Dex) administration led to reduced neutrophil infiltration, decreased pulmonary oedema, and improved lung function in a saline lavage-induced lung injury model. Qin et al. (2019) reported that Dex lowered vascular endothelial growth factor (VEGF) and TNF-α levels while increasing IL-10 expression, stabilising the inflammatory response and protecting against ARDS-related lung damage. These studies collectively highlight GCs' ability to mitigate excessive inflammation, modulate macrophage polarisation, and enhance alveolar fluid clearance (Zhang et al. 2023).
Clinical trials have provided compelling evidence supporting the use of CGs in ARDS. A multicentre randomised controlled trial in Brazil involving COVID-19 patients with moderate-to-severe ARDS demonstrated that Dex significantly increased ventilator-free days (VFDs) compared to standard care alone (Tomazini et al. 2020). Similarly, a study in Spain with 157 ARDS patients found that administering 20 mg/day of Dex from day 1 to day 5 (early administration), followed by a tapering dose, led to reduced inflammatory responses, shorter mechanical ventilation duration, and decreased mortality (Villar et al. 2020).
Hydrocortisone (HC) has also shown promise in clinical settings. A phase III multicentre randomised trial on 800 patients with severe pneumonia revealed that intravenous HC administration significantly lowered 28-day mortality, reduced intubation rates, and decreased vasopressor use (Dequin et al. 2023). A meta-analysis encompassing 702 patients across multiple studies reported that Dex-treated individuals had lower all-cause mortality and mechanical ventilator dependence, without an increase in adverse effects (Feng et al. 2022). Furthermore, a review of 19 studies on ARDS related to severe acute pancreatitis found that 13 studies confirmed CG benefits, with nine suggesting prolonged intravenous administration (≥7 days) as optimal (Meduri et al. 2020). Another network meta-analysis indicated that low-dose MP was the most effective regimen, while high-dose MP or the absence of CGs yielded inferior outcomes (Yoshihiro et al. 2022).
The Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM) have endorsed CG use in ARDS management. The 2017 guidelines recommended MP (1 mg/kg/day for 14 days) for moderate ARDS cases (PaO₂/FiO₂ ratio < 200) (Annane et al. 2017). The 2024 update continues to advocate for CG use in sepsis, septic shock, ARDS, and severe pneumonia, though specific treatment protocols remain undefined (Chaudhuri et al. 2024).
Despite their efficacy, CGs pose risks, particularly with prolonged or high-dose usage. Adverse effects include immunosuppression, hyperglycaemia, femoral head necrosis, cardiovascular complications, and gastrointestinal ulcers (Narum et al. 2014). Moreover, some patients exhibit glucocorticoid resistance, limiting treatment effectiveness. To address these concerns, ongoing research focuses on selective glucocorticoid receptor agonists and modulators (SEGRAMs), which aim to retain anti-inflammatory properties while minimising side effects. Nanotechnology-based CG formulations targeting specific cell types are also being explored to enhance therapeutic efficacy and reduce systemic toxicity (Reichardt et al. 2021).
CGs remain a cornerstone in the management of sepsis-induced ARDS, with substantial evidence supporting their role in mitigating lung inflammation and improving clinical outcomes. However, balancing their benefits against potential adverse effects necessitates careful patient selection and monitoring. Future research should focus on optimising treatment regimens, exploring novel CG formulations, and addressing resistance mechanisms to maximise therapeutic efficacy while minimising risks.
2. Vitamin C
Vitamin C (L-ascorbic acid) is an essential water-soluble vitamin that the human body cannot synthesise and must therefore be obtained through dietary intake. Research has demonstrated that vitamin C possesses significant anti-inflammatory properties at the molecular level and may help mitigate acute damage to the lungs and other organs (Fowler 2024). Pharmacokinetic studies have established that intravenous administration of vitamin C bypasses bioavailability limitations imposed by transport proteins, achieving plasma concentrations up to 70 times higher than those obtained through oral ingestion (Padayatty et al. 2004). Numerous studies have demonstrated that intravenous administration of vitamin C can reduce inflammatory damage associated with sepsis-induced ARDS.
Animal studies have indicated an increase in neutrophil extracellular traps (NETs) within the lung tissue of mice with sepsis. These NETs exhibit strong pro-inflammatory properties, exacerbating lung inflammation and tissue damage. High-dose vitamin C has been shown to mitigate inflammation in sepsis-associated acute lung injury (ALI) by inhibiting the synthesis of NETs and promoting their degradation, thereby improving survival rates and prognosis (Mohammed et al. 2013; Qiao et al. 2022). In a sepsis-induced ALI model triggered by peritoneal injection of faecal solution, mice receiving high-dose intravenous vitamin C exhibited reduced levels of the inflammatory cytokine HMGB1 and chemokine CXCL in plasma and bronchoalveolar lavage fluid (BALF), along with lower protein concentrations in BALF and decreased pulmonary oedema. Further investigations demonstrated that intravenous vitamin C significantly inhibited NF-κB activation, thereby reducing lung inflammation and injury in mice (Fisher et al. 2012). Another study using a faecal solution-induced peritonitis model found that vitamin C treatment led to a substantial reduction in plasma inflammatory cytokine levels and lung tissue pathology scores, further supporting its anti-inflammatory role in sepsis-induced ALI (Canbolat et al. 2022).
Clinical studies have revealed that the majority of sepsis patients exhibit vitamin C deficiency (Carr et al. 2017). In early-stage sepsis, plasma vitamin C levels have been found to be inversely correlated with multiple organ dysfunction scores, likely due to inflammation impairing vitamin C absorption and accelerating its depletion (Borrelli et al. 1996). The CITRIS-ALI trial, a multicentre, double-blind, randomised study conducted across seven intensive care units (ICUs) in the United States, enrolled 167 patients with sepsis-associated ARDS. This trial found that a 96-hour intravenous vitamin C regimen did not lead to significant improvements in organ dysfunction scores or notable changes in markers of inflammation and vascular injury. However, statistically significant differences were observed in secondary outcomes, including the 28-day all-cause mortality rate, ICU ventilator-free days (VFDs), and hospital VFDs (Fowler et al. 2019). Subsequent investigations on plasma biomarkers of CITRIS-ALI trial participants revealed that those receiving intravenous vitamin C exhibited lower levels of cell-free DNA and syndecan-1 in plasma, suggesting that vitamin C may reduce mortality by regulating NET synthesis and degradation while reinforcing the vascular endothelial barrier.
During the COVID-19 pandemic, a clinical trial involving 76 patients found that high-dose intravenous vitamin C infusion significantly lowered inflammatory biomarkers, improved oxygenation, and reduced mortality (Gao et al. 2021). In recent years, clinicians have increasingly adopted a therapeutic regimen combining vitamin C, hydrocortisone, and thiamine to manage severe inflammatory conditions, particularly sepsis and ARDS (Marik et al. 2017). Current evidence indicates that intravenous vitamin C infusion may be safe for critically ill individuals, particularly those with sepsis, with no serious adverse events reported (Koekkoek and van Zanten 2016). However, the most recent study indicates that certain subpopulations may still require further investigation, as there is a possibility that the treatment could be harmful in some cases (Lamontagne et al. 2022).
In conclusion, vitamin C exhibits significant potential as a therapeutic agent in the treatment of sepsis-related lung injury. Its broad range of biological functions, including antioxidant and anti-inflammatory properties, enhancement of microcirculation, and regulation of coagulation, make it a promising candidate for therapy. However, additional clinical studies are necessary to determine the optimal dosage and treatment regimen for long-term effectiveness.
3. Inhibitors of Neutrophil Extracellular Traps (NETs)
In sepsis-induced ARDS, neutrophil activation, migration, adhesion, and infiltration are crucial steps in disease progression. Activated neutrophils release various mediators, including cytokines, reactive oxygen species (ROS), antimicrobial peptides, chemokines, and neutrophil extracellular traps (NETs). NETs, composed of nucleic acids, histones, neutrophil elastase (NE), myeloperoxidase (MPO), and granular proteins, serve a critical role in pathogen entrapment and elimination. However, excessive NET accumulation exacerbates inflammation, promotes tissue damage, and further stimulates NET synthesis, aggravating disease severity (Zhou et al. 2024). Therapeutic strategies for sepsis-induced ARDS focus on promoting NET degradation and inhibiting its synthesis. Deoxyribonuclease I (DNase I) degrades NETs by hydrolysing their DNA scaffold and has been clinically applied (Jorch and Kubes 2017). NET formation can be inhibited by targeting key enzymes such as peptidylarginine deiminase (PAD) and neutrophil elastase (NE). Among NE inhibitors, sivelestat has gained clinical relevance (Matera et al. 2023).
Sivelestat, a low-molecular-weight NE inhibitor, attenuates neutrophil activation and infiltration, reduces inflammatory mediator release, and protects lung tissue from injury. Studies have demonstrated its protective effects in acute lung injury (ALI) models and clinical trials (Chai et al. 2013). Fei et al. (2024) administered intraperitoneal sivelestat in lipopolysaccharide (LPS)-induced ALI mice, demonstrating a reduction in NET formation, ferritin deposition, and ferroptosis. Additionally, sivelestat preserved endothelial barrier integrity, restored hepatocyte growth factor (HGF)/c-MET signalling, and activated downstream PI3K/AKT pathways, mitigating lung damage and improving survival rates. Furthermore, sivelestat preserved the endothelial glycocalyx and maintained endothelial structural integrity in septic ALI mice (Suzuki et al. 2019). Network pharmacology studies identified potential targets and signalling pathways involved in ALI/ARDS treatment with sivelestat, with experimental validation confirming its protective effects via the PI3K/Akt/mTOR pathway by suppressing apoptosis and inflammatory mediator release (Ren et al. 2024).
Clinical trials have corroborated sivelestat’s efficacy in sepsis-related ARDS, particularly in cases secondary to abdominal infections. It has demonstrated improved oxygenation and reduced multi-organ dysfunction (Tsuboko et al. 2012). A large-scale analysis of 4,276 patients from the 2012 Japanese administrative database indicated significantly reduced three-month mortality rates in the sivelestat-treated group (Kido et al. 2017). A clinical study from January 2019 to December 2021 at Wuhan Union Hospital reported significantly decreased serum cytokine levels, including IL-6, IL-8, TNF-α, and HMGB1, in 70 sepsis-related ARDS patients, thus alleviating inflammation (Lv et al. 2024). Another study found reductions in C-reactive protein (CRP), IL-6, and procalcitonin levels after three and seven days of sivelestat treatment, highlighting its anti-inflammatory properties (Che et al. 2024). Safety evaluations included a double-blind trial with 128 healthy volunteers in China, confirming tolerability across incremental dosing regimens (Li et al. 2024).
Despite these findings, concerns persist regarding sivelestat’s overall efficacy. While it improved oxygenation and inflammatory markers, its impact on mortality rates, mechanical ventilation duration, and ICU stay was limited (Pu et al. 2017). These inconsistencies may stem from patient heterogeneity and necessitate further validation in larger ARDS subpopulations.
During the COVID-19 pandemic, sivelestat received emergency approval due to its potential ARDS benefits. However, the precise interplay between NETs and sepsis-related ARDS remains incompletely understood. Further studies are required to elucidate sivelestat’s pharmacological mechanisms, optimal administration protocols, dosing strategies, and overall therapeutic efficacy.
4. Statins
Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, regulate cholesterol synthesis and exhibit anti-inflammatory properties, potentially mitigating acute lung injury (ALI) and sepsis-related acute respiratory distress syndrome (ARDS) (Quist-Paulsen 2010). Preclinical studies suggest that statins protect against ALI by enhancing endothelial barrier function and modulating inflammatory responses (Fessler et al. 2005). Pravastatin reduced pulmonary inflammation, oedema, and hyaline membrane formation in bleomycin-induced ALI models (Woo Kim et al. 2010). Simvastatin suppressed macrophage activation by downregulating inducible nitric oxide synthase (iNOS) and IL-6 in vitro and reduced inflammatory cytokines (IL-6, TNF-α, IL-1β) and neutrophil infiltration in vivo (Haute et al. 2023). Additionally, simvastatin mitigated alveolar epithelial cell apoptosis via the Survivin/NF-κB/p65 pathway in a dose-dependent manner (Nežić et al. 2022).
Clinical studies indicate potential benefits. A cohort study of 575 critically ill patients demonstrated that statins reduced sepsis and ARDS incidence and progression (O’Neal et al. 2011). Simvastatin pre-treatment lowered inflammatory mediators (MPO, TNF-α, matrix metalloproteinases, CRP) and NF-κB expression, exerting systemic anti-inflammatory effects (Nežić et al. 2022). A phase II trial reported reduced cytokine levels, improved organ function, and no significant adverse effects (Craig et al. 2011). However, the REMAP-CAP trial found simvastatin increased organ support-free days but failed to meet superiority thresholds, with elevated creatine kinase levels (Hills et al. 2023). Additionally, a multicentre trial showed rosuvastatin had no mortality benefit and was linked to increased hepatic and renal failure risk (Truwit et al. 2014). Further trials are needed to optimise dosing and evaluate long-term safety.
Conclusion
Sepsis-induced ARDS remains a significant challenge in critical care medicine, with high morbidity and mortality rates. Current therapeutic approaches primarily focus on supportive care, as no specific pharmacological treatment has been definitively established. However, emerging evidence supports the role of anti-inflammatory agents such as corticosteroids and vitamin C in mitigating the excessive immune response that drives ARDS pathophysiology.
Conflict of Interest
None.