ICU Management & Practice, Volume 22 - Issue 3, 2022
Delirium: How Can We Protect Our Patients? Detection and Treatment Strategies
In this article, the authors aim to summarise the current management of delirium, emphasising new publications and possible new studies that will shed light on delirium management strategy.
Introduction
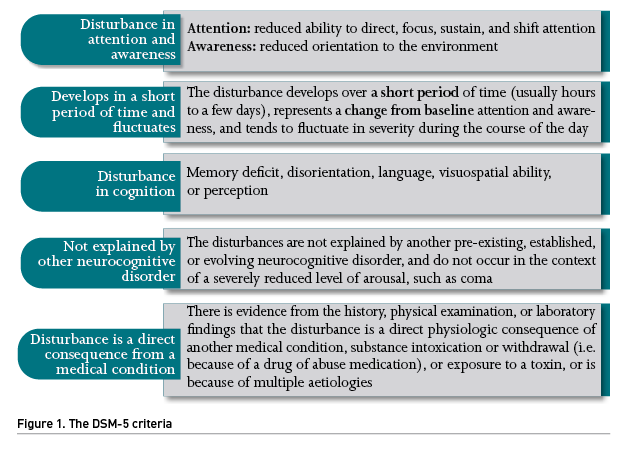
According to an updated nomenclature of delirium after an interdisciplinary panel of experts from ten medical societies (Slooter et al. 2020), delirium refers to a clinical state characterised by a combination of features defined by the DSM-5 criteria (Figure 1). The panel also recommended using the term sub-syndromal delirium for acute cognitive changes that are compatible with delirium but do not fulfill all DSM-5 delirium criteria. On the other hand, Patel et al. (2014) described a rapidly reversible sedation-related delirium, defined as delirium while receiving sedation that resolved within two hours after stopping sedation during a spontaneous awakening trial.
There is an attempt to categorise the delirium spectrum into subphenotypes, using psychomotor subtypes (known as hypoactive, hyperactive, and mixed) or inflammatory/non-inflammatory delirium. Identifying specific subphenotypes would improve our understanding of the relationship between the clinical symptoms and pathophysiology, suggesting a progression from subphenotypes to endotypes, setting a biological–clinical subtype hybrid (Bowman et al. 2021).
For several years now, and thanks to the efforts of the community of healthcare professionals dedicated to the care of critically ill patients, the attention devoted to delirium has increased considerably, with a steady increase in publications related to its epidemiology, diagnosis, prevention, and treatment. Delirium incidence, once reported in 60–80% of mechanically ventilated patients, is down by about 25% in many ICUs worldwide (Gibb et al. 2020; Stollings et al. 2021).
Delirium development is associated with multiple complications (especially in hypoactive motoric subtype): increased mortality, longer duration of mechanical ventilation, higher reintubation rate, increased hospital stay, higher early instrumental activities of daily living dependence scores, and worse long-term cognition (Salluh et al. 2015; Ely et al. 2017; Girard et al. 2018; Hayhurst et al. 2020; Rengel et al. 2021; Hughes et al. 2021). Since effective treatment of delirium has proven troublesome, prophylactic strategies have become paramount. In this sense, a comprehensive study of the risk factors associated with this clinical entity would facilitate high-risk patients’ detection. In recent reviews, factors related to the risk of developing delirium (Zaal et al. 2015) were advanced age, personal history of previous high blood pressure or cognitive impairment, urgent surgery or trauma before admission to the ICU, APACHE II upon admission, and need for mechanical ventilation. A recent study found that the highest risk observed for developing delirium clustered in patients who presented more than two organ failures and patients over 74 years old (Lobo-Valbuena et al. 2021).
Detection and Delirium Severity
Routine monitoring of delirium, using validated scales (such as CAM-ICU or ICDSC), is a good practice statement according to the latest Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU Guidelines (Devlin et al. 2018), and is a strong recommendation with moderate-quality evidence according to the Pan-American and Iberian Federation of Societies of Critical Medicine and Intensive Therapy (Celis-Rodriguez et al. 2020). According to published data, the CAM-ICU has a sensitivity of around 93%, specificity of 98%, and high interrater reliability (κ= 0.96; 95% CI, 0.92–0.99) (Ely et al. 2001). Regarding ICDSC, it demonstrated a pooled sensitivity of 74% and specificity of 82% (Gusmao-Flores et al. 2012).
Moreover, both assessment tools showed similar moderate-to-good statistical performance, supporting either for early prediction model or recalibrated prediction model (Wassenaar et al. 2019). Both should be used at least once per shift and when the patient’s clinical situation (primarily neurological) presents an abrupt change. We must remind that the usefulness of both tools requires training of the health professionals that use them.
Concerning delirium severity, we may use DRS, DRS-R-98, CAM-S, and the CAM-ICU-7 (Trzepacz 1999; Inouye et al. 2014; Khan et al. 2017). A recent study (Krewulak et al. 2020) compared CAM-ICU-7 with ICDSC as measures of the spectrum of delirium severity in critically ill adult patients. The CAM-ICU-7 and ICDSC had a significant positive correlation (0.58, p<0.001), with a moderate agreement between the tools as measures of delirium (kappa = 0.51).
We must highlight that nearly all the clinical trials investigating strategies to prevent and/or treat delirium are based on delirium assessment tools, with the risk that entails (false-positive or negative screening or work overload for healthcare staff). The ability of assessment tools to improve patient outcomes could be associated with the intensity of the training strategy used and the quality improvement initiatives deployed, which points out the need of promoting training for healthcare professionals, family members, and lay population alike (Gélinas et al. 2011; Radtke et al. 2012). A recent study (Fiest et al. 2020) explored the idea of family-administered delirium detection: Family Confusion Assessment Method AUROC was 65.0% (95% CI, 60.0–70.0%), 71.0% (95% CI, 66.0–76.0%) for possible delirium (cutpoint of 4) on the Sour Seven and 67.0% (95% CI, 62.0–72.0%) for delirium (cutpoint of 9) on the Sour Seven. These AUROC were lower than the standard of care (ICDSC or CAM-ICU). Adding the FAM-CAM and Sour Seven to the standard of care improved sensitivity at the expense of specificity.
In recent years, several models have been developed to predict the risk of ICU delirium based on known risk factors for this condition. The prediction of delirium in ICU patients (PRE-DELIRIC) model (van den Boogard et al. 2012) was developed to predict patients’ risk of delirium from some clinical features present in the first 24 hours of ICU admission. This tool displayed good discriminative ability and was later recalibrated in an international multicentre trial (van den Boogard et al. 2014). The early PRE-DELIRIC (E-PRE-DELIRIC) (Wassenaar et al. 2015) was subsequently developed to predict patients’ risk of delirium at the time of ICU admission but showed a lower discriminative ability than the previous models. An alternative, referred to as the Lanzhou model, also relies on several features present in the first 24 hours of ICU admission. Green et al. (2019) found that the PRE-DELIRIC [AUROC curve 0.79 (95% CI, 0.75– 0.83)], the recalibrated PRE-DELIRIC [AUROC curve 0.79 (95% CI, 0.75– 0.83)], and Lanzhou model [AUROC curve of 0.77 (95% CI, 0.72–0.81)] performed comparably to the original validation studies, and that the e-PRE-DELIRIC [AUROC curve of 0.72 (95% CI, 0.67–0.77)] displayed moderate predictive ability.
Other studies that try to shed some light on the diagnosis of delirium imply the use of magnetic resonance imaging and neurophysiological studies. Pre-operative deep and white matter and thalamic abnormalities on diffusion tension imaging appeared in elderly patients with postoperative delirium (Shiori et al. 2010). On the other hand, EEG slowing, an increase of delta (1–4 Hz) and/or theta power (4–8 Hz), or a decrease of alpha power (8–12 Hz), correlates with the presence of delirium across various types of delirium presentations (odds ratio 10.3, 95% CI 5.3–20.1) (van der Kooi et al. 2015; Kimchi et al. 2019; Boord et al. 2021). Efforts to merge the information provided by the physical examination (through validated scales) and the information provided by some test (in this case, EEG) has led to the publication of the Electroencephalographic Confusion Assessment Method Severity Score (E-CAM-S) (van Sleuwen et al. 2021). This study used CAM short form and CAM-S to assess delirium presence and severity, respectively; they afterward calculated the E-CAM-S using four frontal EEG channels. 373 patients were analysed: E-CAM-S reliably quantified delirium severity (it successfully correlated with clinical CAM-S scores with an R = 0.68; p < 0.001) and was independently associated with hospital length-of-stay (correlation with LOS: E-CAM-S, 0.33; CAM-S, 0.41; p = 0.082) and in-hospital mortality (AUROC: E-CAM-S 0.77 [0.72–0.82] CAM-S 0.81 [0.75–0.85]; p = 0.188) across a wide range of acutely hospitalised adults. There is still a long way to go before an accurate diagnosis (through imaging or EEG) of delirium in critically ill patients can be made.
Prevention Strategies
A wide-ranging list of prevention strategies evaluated includes pharmacological, sedation, and non-pharmacological single or multi-component intervention. However, to date, no known effective intervention has shown a significant decrease in the incidence of delirium.
Pharmacological interventions
One of the last published systematic reviews and meta-analyses of pharmacological interventions (Burry et al. 2021) found that dexmedetomidine could reduce the odds of delirium occurrence relative to placebo (OR 0.43, 95% CI 0.21–0.85; moderate certainty). It was the only identified intervention that could probably reduce the length of ICU or hospital stay relative to placebo. It could also do so relative to antipsychotics, but with less certainty. The study concluded with three take-home messages: (1) compared to placebo or benzodiazepines, dexmedetomidine probably prevents delirium; (2) a sedation-minimisation strategy that targets reduced exposure to sedatives might prevent delirium; and (3) antipsychotics may not prevent delirium. Despite the negative results, we are still trying to find a drug that could prevent, at least in part, the development of delirium in the critically ill patient.
A post hoc analysis of the REDUCE trial (prophylactic haloperidol use for delirium in ICU patients at high risk for delirium; (van den Boogaard et al. 2018) assessed the association between treatment haloperidol exposure and mortality in a population of critically ill adults without delirium at the time of ICU admission (Duprey et al. 2021). If delirium occurred, treatment with open-label intravenous haloperidol was administered at clinician discretion. They demonstrated that the use of haloperidol to treat incident delirium (defined as “delirium occurring after (and not before) ICU admission”) might be associated with lower 28-day mortality in a dose-dependent, time-dependent manner. Over 28 days of follow-up, each milligram of treatment haloperidol administered daily to a patient with delirium was associated with a 7% decrease in mortality (HR 0.93; 95% CI, 0.91–0.95). Although an association between haloperidol and reduced mortality was observed up to 90 days, it was lower than that observed at 28 days suggesting this effect may wane over time. Indeed, mortality at 90 days among patients with delirium may also be better attributed to the comorbidity burden of patients at baseline rather than the specific delirium care a patient receives during their ICU and post-ICU hospital stay.
Among the latest published protocols and studies, we may find the ProMEDIC study (Prophylactic Melatonin for Delirium in ICU): a multi-centre, randomised, double-blinded, placebo-controlled trial that will determine whether melatonin given prophylactically decreases delirium in critically ill patients (Wibrow et al. 2021).
Non-pharmacological interventions
Non-pharmacologic multi-component strategies have been studied extensively, and most studies suggest these are the most effective methods to prevent delirium, as they use several interventions simultaneously (Deng et al. 2020).
One example of a multi-component strategy is the ABCDEF bundle (Figure 2) (Marra et al. 2017). This bundle improved patient outcomes in several non-randomised studies, turning into a large nationwide collaborative. However, to our knowledge, there is currently not a single RCT demonstrating the benefit of the ABCDEF bundle, which is the gold standard for demonstrating therapeutic efficacy. Moreover, a recent meta-analysis (Zhang et al. 2021) has failed to support that bundle interventions effectively reduce ICU delirium prevalence and duration. However, they seem to be effective in lowering the proportion of patient-days with coma, hospital length of stay, and 28-day mortality.
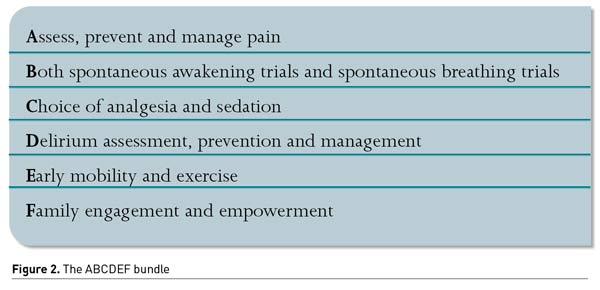
Recently, an expert panel proposed to update the bundle adding an “R” for respiratory drive control. The objectives are (1) reducing sedatives (especially benzodiazepines and opioids), (2) preferring more participative ventilation modes, and (3) optimising management of patient’s associated factors of high respiratory demand (metabolic acidosis, fever, pain, anxiety, dyspnoea) (Chanques et al. 2020). Optimisation of ventilator settings should be a priority.
Treatment Strategies
Up to our knowledge, no single pharmacological agent can treat delirium. Haloperidol, atypical antipsychotics, and other alternative therapies have been thoroughly studied, but we are still far from identifying a silver bullet. PADIS guidelines suggest against routine use of drugs to treat delirium. Nevertheless, the guidelines do point out the need for these drugs to manage agitation or stress-related symptoms. It is essential to realise that it is not treating delirium. We should use the smallest doses and the shortest possible duration.
A retrospective study (Boncyk et al. 2021) tried to describe the prescribing practices for the management of ICU delirium. 45.6% of the patients received pharmacological treatment, including 45.4% receiving antipsychotics. Haloperidol, olanzapine, and quetiapine comprised more than 97% of used antipsychotics, with 48% of the patients receiving two or more and 20.6% continued antipsychotic medications at hospital discharge. Haloperidol and olanzapine were associated with greater odds of continued delirium and increased hazard of in-hospital mortality, while quetiapine showed a decreased risk of in-hospital mortality. Haloperidol, olanzapine, and quetiapine were associated with fewer days alive and free of hospitalisation (P < .001). What conclusions can we draw out? These medications may not portend benefit, may introduce additional harm, and should be used with caution for delirium management. Furthermore, the continuation of these medications through hospitalisation and discharge questions their safety and role in patient recovery.
Within the past decade, it has become evident that antipsychotics do not diminish the risk of ICU delirium, nor do they improve the associated adverse outcomes. Studies, such as the HOPE-ICU study (Page et al. 2013), the HARPOON study (Schrijver et al. 2018), the MIND study (Girard et al. 2010), and the MIND-USA study (Girard et al. 2018) have not found differences when using different antipsychotics. Moreover, a recent systematic review of antipsychotics for treating delirium in hospitalised adults found no difference among haloperidol, atypical antipsychotics, and placebo in terms of delirium duration, hospital length of stay, or mortality (Nikooie et al. 2019).
Regarding the use of other drugs, such as α-2 adrenoreceptor agonists, a randomised, double-blind placebo-controlled trial did not impact ICU or hospital length of stay when using dexmedetomidine (Reade et al. 2016). Conversely, a recent systematic review and meta-analysis (Liu et al. 2021), assessing the role of dexmedetomidine in the treatment of delirium in critically ill patients, showed a reduced duration of delirium to a greater extent than did the placebo (just in one study), a lower-point prevalence of delirium after treatment (OR 0.39; 95% CI, 0.20, 0.76; P=0.006) and a shorter time to resolution of delirium compared with those of other drugs (including haloperidol).
To add fuel to the fire, a recent study (Smit et al. 2021), analysing haloperidol and clonidine in agitated delirious patients, concluded that the use of both drugs in delirious ICU patients could be associated with a reduced probability of delirium resolution. In this case, the likelihood of delirium resolution was lower in delirious patients who received haloperidol (OR 0.47, 95% CI 0.39–0.57), clonidine (OR 0.78, 95% CI 0.63–0.97), or both (OR 0.45, 95% CI 0.36–0.56) compared to untreated delirious patients. Delirious patients who received haloperidol, clonidine, or both generally had longer delirium duration, more delirium and ventilation days, and longer hospital length of stay than untreated delirious patients. These agents did not affect ICU mortality. Other studied drugs, such as statins or ketamine, have ended up with negative results in several randomised controlled trials.
Last Comments
One of the main problems of the studies carried out in this field is the high heterogeneity when publishing results, which hinders a strict and critical evaluation of the published studies. An updated systematic review, focused on the design and analysis of delirium outcomes, identified 65 RCTs conducted among ICU patients with a delirium-related primary outcome. Most of these RCTs were delirium prevention trials, with considerable heterogeneity in the maximum duration of participant follow-up and whether delirium assessments occurred after ICU discharge. Eight unique statistical methods were used to detect differences in delirium incidence across intervention groups. Heterogeneity in statistical methods was similar across the two central populations of patients enrolled in delirium RCTs; surgery and critically ill patients. Therefore, creating uniform standards for statistical analyses and reporting in delirium RCTs could improve the quality of individual trials and the ability to harmonise results across trials (Coulantoni et al. 2021).
An international effort was made to develop a COS (Core Outcome Set) appropriate for clinical trials of interventions designed to prevent and/or treat delirium for critically ill adults (Rose et al. 2021). A COS is an agreed-upon minimum set of outcomes to be measured and reported in “all” studies relating to a specific health condition or intervention. After following the Core Outcome Measures in Effectiveness Trials (COMET) guidelines, seven outcomes were selected for the COS (Figure 3).
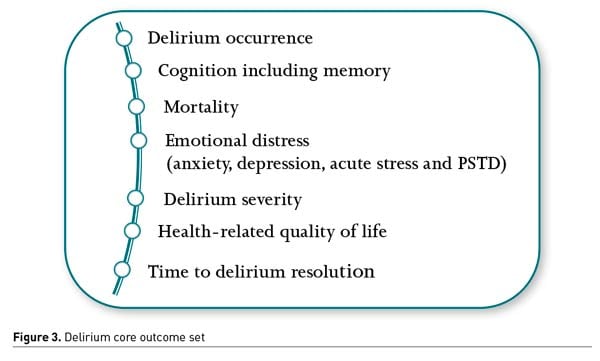
COS development enhances research relevance and patient-centredness and may facilitate a more rapid understanding of effective treatments and their adoption into clinical practice. Adopting delirium COS as part of future research protocols would also improve the homogeneity of reported outcomes, increasing statistical power, and precision of meta-analyses. It would open doors for evidence-based decisions, improving the clinical care of critically ill adults.
Delirium patients show an increased risk of developing post-ICU syndrome, already known to affect patients’ perceived quality of life profoundly (Needham et al. 2012; van der Schaaf et al. 2015). Improving our understanding of risk factors amenable to intervention could improve our clinical management, plus develop post-ICU care programmes. In our case, after a deep statistical analysis of our ICU admitted patients (Lobo-Valbuena et al. 2021), and thanks to the great collaboration of our nursing team, high-risk patients are closely followed-up once discharged. This has led to our first multidisciplinary protocol for managing post-ICU syndrome (coordinating both the hospital team and the primary care health centres attached to the hospital area to which we belong).
After one year of implementation of the protocol, we observed improvement in some mental health components (fear, self-esteem, coping, sleep disorders) and the patient’s ability to perform basic activities of daily living (measured by the Barthel index). Positive results on the Zarit scale (measuring caregiver overload) would also stem from the high support perceived by the patients’ families and relatives (Lobo-Valbuena et al. 2021). The management of complex patients at risk of PICS requires a multidisciplinary approach, both inside and outside the hospital. For many ICU survivors, discharge from the hospital marks the beginning of an uphill struggle.
Finally, the overall management of delirium in critically ill patients should not lag. We should try to seek new tools in the new and promising technologies. For example, a new study protocol published a few months ago will try to assess the use of virtual reality stimulation as part of the multi-component strategies (Naef et al. 2021). There have already been publications regarding this topic, showing the feasibility, usability, and acceptance of virtual reality stimulation as a new non-pharmacological intervention to comfort patients during their stay in the ICU (Gerber et al. 2017; Gerber et al. 2019). On the other hand, machine learning could predict delirium, especially in the postoperative population (Wang et al. 2020; Fliegenschmidt et al. 2021). In another published study (Davoudi et al. 2019), pervasive monitoring and machine learning were continuously used to assess delirium and agitation. Camera and accelerometers were employed to record facial expressions and movements, and a pre-trained neural network was used for facial recognition and expression detection through single elements.
We cannot rest on our laurels. The impact of delirium on the critically ill patient, both short and long-term, compels us to keep searching and investigating. Only then will we be able, at some point, to reduce the incidence and prevalence of delirium. Only then may we find a new solution or new drug that will help our delirious patients, reducing the physical, emotional, and social impact associated with delirium.
Abbreviations
APACHE II Acute Physiology and Chronic Health Disease Classification System II
AUROC Area under the receiver operating characteristic
CAM-ICU Confusion Assessment Method for the ICU
CAM-ICU-7 Confusion Assessment Method for the ICU 7 (points) delirium severity scale
CAM-S Confusion Assessment Method – Severity Scale
COS Core Outcome Set DRS Delirium Rating Scale
DRS-R-98 Delirium Rating Scale-Revised 98 assessments
DSM Diagnostic and statistical manual of mental disorders
EEG Electroencephalogram
E-PRE-DELIRIC Early prediction of delirium in ICU patients
HR Hazard ratio
ICDSC Intensive Care Delirium Screening Checklist
ICU Intensive care unit OR Odds ratio
PRE-DELIRIC Prediction of delirium in ICU patients
RCT Randomised controlled trial
Conflict of Interest
F Gordo has performed consultancy work and formation for Medtronic. The other authors have no competing interests.
«« Clinical, Organisational Factors and COVID-19 Mortality
Nutrition Therapy and Clinical Outcomes in Critically Ill Patients »»
References:
Boncyk CS, Farrin E, Stollings JLet al (2021) Pharmacologic Management of Intensive Care Unit Delirium: Clinical Prescribing Practices and Outcomes in More Than 8500 Patient Encounters. AnesthAnalg. 133:713-22.
Boord MS, Moezzi B, Davis D et al (2021) Investigating how electro- encephalogram measures associate with delirium: A system- atic review. Clin Neurophysiol. 132:246-257.
Bowman EML, Cunningham EL, Page VJ et al (2021)Phenotypes and subphenotypes of delirium: a review of current categorisations and suggestions for progression. Crit Care. 25:334.
Burry LD, Cheng W, Williamson DRet al (2021) Pharmacological and non-pharmacological interventions to prevent delirium in critically ill patients: a systematic review and network meta-analysis. Intensive Care Med. 47:943-960.
Celis-Rodriguez E, Díaz Cortes JC, Cárdenas Bolívar YR et al (2020)Evidence-based clinical practice guidelines for the management of sedoanalgesia and delirium in critically ill adult patients. Med Intensiva. 44(3):171-184.
Chanques G, Constantin JM, Devlin JW et al (2020) Analgesia and sedation in patients with ARDS. Intensive Care Med. 46(12):2342-2356.
Coulantoni E, Koneru M, Akhlaghi Net al. (2021)Heterogeneity in design and analysis of ICU delirium randomized trials: a systematic review. Trials. 22(1):354.
Davoudi A, Malhotra KR, Shickel Bet al. (2019) Intelligent ICU for autonomous patient monitoring using pervasive sensing and deep learning. Sci Rep. 9(01):8020.
Deng LX, Cao L, Zhang LN et al (2020) Non-pharmacological interventions to reduce the incidence and duration of delirium in critically ill patients: A systematic review and network meta-analysis. J Crit Care. 60:241-248.
Devlin JW, Skrobik Y, GélinasCet al. (2018)Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 46(9):e825-e873.
Duprey MS, Devlin JW, van derHoeven JG et al (2021)Association Between Incident Delirium Treatment With Haloperidol and Mortality in Critically Ill Adults. Crit Care Med. 49(8):13030-1311.
Ely EW, Inouye SK, Bernard GR et al (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU)JAMA. 286(21):2703-2710.
Ely EW, Shintani A, Truman Bet al.. (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 291(14):1753-62.
Fiest KM, Krewulak KD, Ely EWet al. (2020) Partnering with family members to detect delirium in critically ill patients. Crit Care Med. 48(7):954-961.
Fliegenschmidt J, Hulde N, PresingMGet al. (2021) Artificial intelligence predicts delirium following cardiac surgery: a case study. J Clin Anesth. 75:110473.
Gélinas C, Arbour C, MichaudCet al. (2011)Implementation of the Critical- Care Pain Observation Tool on pain assessment/management nursing practices in an intensive care unit with nonverbal critically ill adults: A before and after study. Int J Nurs Stud. 48:1495-1504.
Gerber SM, Jeitziner MM, KnobelSEet al. (2019) Perception and performance on a virtual reality cognitive stimulation for use in the intensive care unit: a non-randomized trial in critically ill patients. Front Med. 6.
Gerber SM, Jeitziner MM, SängerSDet al. (2019) Comparing the relaxing effects of different virtual reality environments in the intensive care unit: observational study. JMIR PerioperMed. 2(2): e15579.
Gerber SM, Jeitziner MM, Wyss Pet al. (2017) Visuo-acoustic stimulation that helps you to relax: a virtual reality setup for patients in the intensive care unit. Sci Rep. 2017.7(1):1-0.
Gibb K, Seeley A, Quinn Tet al. (2020) The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing. 49:352-60.
Girard TD, Exline MC, Carson SSet al. (2018) Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 379(26):2506-2516.
Girard TD, Pandharipande PP, Carson SSet al. (2010) Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 38(2):428-437.
Girard TD, Thompson JL, Pandharipande PPet al. (2018) Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 6(3):213-222.
Green C, Bonavia W, Toh C et al (2019) Prediction of ICU delirium: validation of current delirium predictive models in routine clinical practice. Crit Care Med. 47(3):428-435.
Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC (2012)The confussion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care.16(4):R115.
Hayhurst CJ, Marra A, Han JHet al. (2020)Association of hypoactive and hyperactive delirium with cognitive function after critical illness. Crit Care Med. 48(6):e480-488.
Hughes CG, Hayhurst CJ, Pandharipande Pet al. (2021) Association of Delirium during Critical Illness With Mortality: Multicenter Prospective Cohort Study.Anesth Analg. 133(5):1152-1161.
Inouye SK, Kosar CM, Tommet D et al (2014) The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 160(8):526-533.
Khan BA, Perkins AJ, Gao Set al. (2017) The confusion assessment method for the ICU-7 delirium severity scale: a novel delirium severity instrument for use in the ICU. Crit Care Med. 45(5):851-857.
Kimchi EY, Neelagiri A, Whitt Wet al. (2019) Clinical EEG slowing correlates with delirium severity and predicts poor clinical outcomes. Neurology. 93(13):e1260-e1271.
Krewulak KD, Rosgen BK, Ely EW et al (2020) The CAM-ICU-7 and ICDSC as measures of delirium severity in critically ill adult patients. PLoS One.15(11):e0242378.
Liu X, Xiong J, Tang Yet al. (2021)Role of dexmedetomidine in the treatment of delirium in critically ill patients: a systematic review and meta-analysis. Minerva Anestesiol. 87:65-76.
Lobo-Valbuena B, Gordo F, Abella Aet al. (2021)Risk factors associated with the development of delirium in general ICU patients. A prospective observational study. PLos One.16(9):e0255522.
Lobo-Valbuena B, Sánchez Roca MD, Regalón Martin MPet al. (2021)Post-intensive care syndrome: ample room for improvement. Data analysis after one year of implementation of a protocol for prevention and management in a second-level hospital. Med Intensiva. 45(8):e43-e46.
Marra A, Ely EW, Pandharipande P et al (2017) The ABCDEF Bundle in Critical Care. Crit Care Clin. (33)225-243.
Naef AC, Jeitziner MM, Gerber SMet al. (2021) Virtual reality stimulation to reduce the incidence of delirium in critically ill patients: study protocol for a randomized clinical trial. Trials. 22(1):174.
Needham DM, Davidson J, Cohen Het al. (2012) Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 40(2):502-9.
Needham DM, Davidson J, Cohen Het al. (2012) Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 40(2):502-9.
Nikooie R, Neufeld KJ, Oh ESetal..Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann Intern Med. 171(07):485-49.
Page VJ, Ely EW, Gates Set al. (2013) Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet RespirMed. 1(07):515-523.
Patel SB, Poston JT, Pohlman Aet al. (2014) Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 189(6):658-665.
Radtke FM, Heymann A, Franck Met al. (2012) How to implement moni- toring tools for sedation, pain and delirium in the intensive care unit: An experimental cohort study. Intensive Care Med. 38:1974-1981.
Reade MC, Eastwood GM, Bellomo Ret al. (2016) Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 315(14):1460-1468.
Rengel KF, Hayhurst CJ, Jackson JCet al. (2021) Motoric subtypes of delirium and long-term functional and mental health outcomes in adults after critical illness. Crit Care Med. 49(5):e521-532.
Rose L, Burry L, Agar Met al. (2021) A Core Outcome Set for Research Evaluating Interventions to Prevent and/or Treat Delirium in Critically Ill Adults: An International Consensus Study (Del-COrS) Crit Care Med. 49(9):1535-1546.
Salluh JI, Wang H, Schneider EBet al. (2015) Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 350:h2538.
Schrijver EJM, de Vries OJ, van de Ven PMet al. (2018)Haloperidol versus placebo for delirium prevention in acutely hospitalised older at risk patients: a multi-centre double-blind randomised controlled clinical trial. Age Ageing. 47(01):48-55.
Shioiri A, Kurumaji A, Takeuchi Tet al. (2010) White matter abnormalities as a risk factor for postoperative delirium revealed by diffusion tensor imaging. Am J GeriatPsyc. 18(8):743-753.
Slooter AJ, Otte WM, Devlin JWet al. (2020) Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 46:1020-1022.
Smit L, Dijkstra‐Kersten SMA, Zaal IJ et al (2021) Haloperidol, clonidine and resolution of delirium in critically ill patients: a prospective cohort study. Intensive Care Med. 47:316-324.
Stollings JL, Kotfis K, Chanques Get al. (2021) Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 47:1089-1103.
Trzepacz PT (1999) The delirium rating scale - its use in consultation- liaison research. Psychosomatics 40(3):193-204.
van den Boogaard M, Pickkers P, SlooterAJet al. (2012) Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: Observational multi-centre study. BMJ.344:e420.
van den Boogaard M, Schoonhoven L, MasedaEet al. (2014)Recalibration of the delirium prediction model for ICU patients (PRE-DELIRIC): A multinational observational study. Intensive Care Med. 40: 361-369.
van den Boogaard M, Slooter AJC, Brüggemann RJM et al (2018) Effect of haloperidol on survival among critically ill adutls with a high risk fo delirium: the REDUCE randomized clinical trial. JAMA. 319(7):680-690.
van derKooi AW, Zaal IJ, KlijnFAet al. (2015)Delirium detection using EEG: What and how to measure. Chest. 147:94-101.
van Der Schaaf M, Bakhshi-Raiez F, Van Der SteenMet al. (2015)Recommendations for intensive care follow-up clinics. report from a survey and conference of Dutch intensive cares. Minerva Anestesiol. 81(2):135-44.
van Der Schaaf M, Bakhshi-Raiez F, Van Der SteenMet al. (2015)Recommendations for intensive care follow-up clinics. report from a survey and conference of Dutch intensive cares. Minerva Anestesiol. 81(2):135-44.
van Sleuwen M, Sun H, Echkardt C et al (2021)Physiological Assessment of Delirium Severity: The Electroencephalographic Confusion Assessment Method Severity Score (E-CAM-S) Crit Care Med.
Wang Y, Lei L, Ji Met al. (2020)Predicting postoperative delirium after microvascular decompression surgery with machine learning. J Clin Anesth. 66:109896.
Wassenaar A, Schoonhoven L, Devlin JW et al (2019) External Validation of Two Models to Predict Delirium in Critically Ill Adults Using Either the Confusion Assessment Method-ICU or the Intensive Care Delirium Screening Checklist for Delirium Assessment. Crit Care Med. 47:e827-e835.
Wassenaar A, van den Boogaard M, van Achterberg Tet al. (2015) Multinational development and validation of an early prediction model for delirium in ICU patients. Intensive Care Med. 41:1048-1056
Wibrow B, Martinez FE, Ford A et al (2021) Statistical analysis plan for the Prophylactic Melatonin for Delirium in Intensive Care (ProMEDIC): a randomised controlled trial. Trials. 22(1):7.
Zaal IJ, Devlin JW, PeelenLMet al. (2015) A systematic review of risk factors for delirium in the ICU. Crit Care Med. 43(1):40-7.
Zhang S, Han Y, Xiao Q et al (2021)Effectiveness of bundle interventions on ICU delirium: a meta-analysis. Crit Care Med. 49(2):335-346.