Why Should We Be Measuring Ionized Magnesium?
Magnesium in the Human Body
Magnesium (Mg) is the 4th most abundant cation in the human body. The majority of it is found intracellularly, more than half in bone, about a third in muscle, and the remaining in soft tissue and extracellular fluid (Figure 1). Magnesium is found in the diet from a variety of sources: green leafy vegetables (Mg is found in chlorophyll), nuts and seeds, fruits, yogurt, and unrefined grains such as wheat and oatmeal. It is absorbed in the gut and excreted by the kidneys. Even though it is found in a wide variety of foods, much of it is lost during processing, and the Mg content of fruits and vegetables has decreased by 20-30% over the past 60 years. Thus it is estimated that up to 60% of healthy adults do not get the recommended amount of Mg in their diet.[1] Although only about 1% of total Mg is located extracellularly, this is an extremely important fraction, as Mg is involved in a multitude of physiologic processes (Table 1), many of which are critical to overall health, including cardiac conduction and contractility, energy production, ion transport, and coagulation.
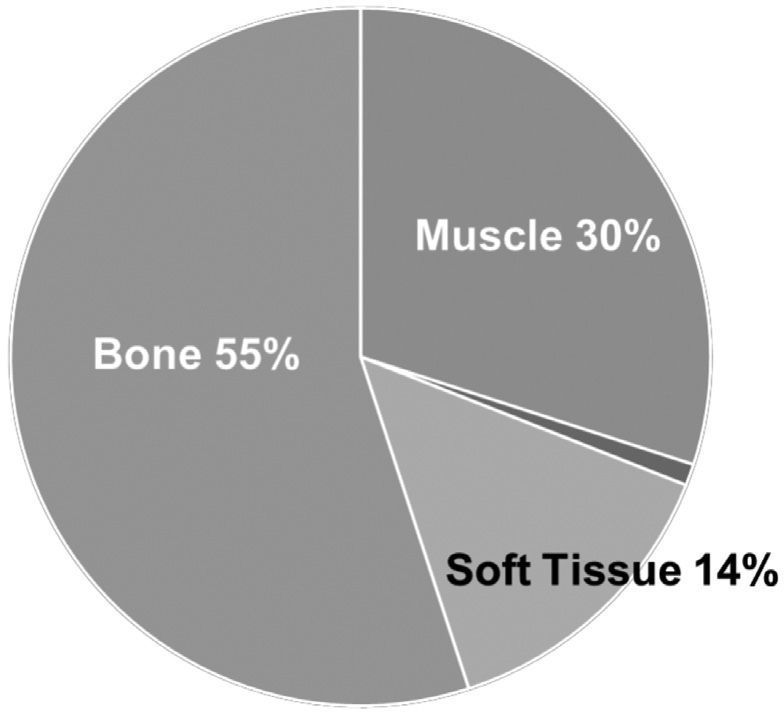
Figure 1: Distribution of Mg in the body
Table 1: Some physiologic functions of Mg

Ionized Magnesium Does Not Always Correlate With Total Magnesium
The portion of extracellular Mg that is physiologically active in these processes is ionized Mg (iMg). Mg in serum is found in 3 states: Protein bound (20-30%), complexed with anions (5-15%), and ionized (55-70%) (Figure 2). Because the amount of bound or complexed Mg can vary significantly, especially in illness, the iMg can vary in an unpredictable way. Conventional laboratory testing typically only measures total serum Mg (tMg), which is often not reflective of iMg. Unlike ionized calcium, which is commonly measured and which is also readily calculated from the total calcium and albumin levels, iMg is neither commonly measured nor easily calculated.[2] In particular, patients in the ICU, perioperative patients, extremes of age (elderly or neonatal), and those with chronic kidney disease can have significant variation in their iMg levels without much change in tMg, or vice versa. This variability can be due to changes in serum protein levels, or the variability of anions in the blood which would cause the iMg level to fluctuate. In a study from Sweden, 25% of patients in the ICU had low iMg despite a normal tMg, and 9% had a high iMg in the presence of a normal tMg (Figure 3).[3]

Figure 2: Extracellular Mg Distribution

Figure 3: Relationship of iMg and tMg in an ICU Cohort [3]

Figure 4: Relationship of tMg and iMg [4]
In another study looking at ICU patients, 30% of tMg measurements did not correlate with iMg; the majority of low tMg were false negatives, potentially leading to over-supplementation with Mg and running up laboratory costs (Figure 4).[4] Other studies have shown similar findings of a 30% discrepancy between tMg and iMg.[5] The authors of this study commented, “Reliable concentrations of serum ionized magnesium can be obtained only by direct measurement and not by calculation from serum total magnesium and albumin.”[5] Thus, it is safe to say that tMg is not reflective of iMg in approximately one-third of patients, and for accurate assessment of functional Mg the iMg is the preferred analyte to measure, especially in critically ill patients. There seems to be no easy way to calculate iMg from tMg.
The data presented above deals with hospitalized patients, but a recent study evaluated healthy individuals following an oral Mg supplement. That study found that iMg was a more sensitive marker for evaluating the acute rise of Mg afteroral intake, and the study is expanding to a larger randomized, controlled trial.[6] In this group, iMg but not tMg rose following oral ingestion of Mg. “we demonstrated the superiority of concentrations of iMg2+ in blood, compared toconcentrations of total magnesium in serum and total urine magnesium content, as a rapid and sensitive measure ofdietary intake of magnesium in healthy humans. The finding that a single dose of 300 mg of magnesium can alter iMg2+,but not total magnesium, suggests that the iMg2+ method is more sensitive”[6]
Consequences of Dysmagnesemia
Hypomagnesemia (usually due to inadequate dietary intake) is more prevalent than hypermagnesemia because thekidneys are very good at excreting Mg until creatinine clearance drops below 10 mL/min.[7] The consequences ofdisordered magnesium, either hypo or hyper, can be significant. Since it is involved in numerous physiologic processes,it can affect many systems. One of the most serious and potentially life-threatening complications of hypomagnesemia iscardiac arrhythmias, due to abnormal conduction and contractility of the heart. In the acute setting, hypomagnesemia can also result in seizures, muscle cramps, migraine headaches, and secondary abnormalities of other electrolytes such aspotassium, sodium, and calcium.[1] Hypermagnesemia is seen in people with severe chronic kidney disease, can be seenin people taking medications with magnesium (typically laxatives), and can cause flushing, bradycardia, hypotension, respiratory depression, and muscle weakness.[1] Magnesium infusions are also used for treating pregnant women in pre-term labor, where tMg levels are typically closely monitored.[8] Low iMg but not tMg has also been shown to be a risk factor for preeclampsia, which again suggests that iMg is the preferred analyte to measure.[9]
There is a significant amount of data to support the fact that either hyper- or hypomagnesemia can affect outcomes. Most studies evaluating outcomes related to Mg have studied tMg, not iMg. One study that did evaluate iMg in ICU patients found that development of ionized hypomagnesemia was an independent predictor of mortality.[10] Additionally, the study found a poor correlation between tMg and iMg, with up to 85% of patients with low tMg having normal iMg.[10] In a large study from the Mayo Clinic, over 280,000 patients were retrospectively evaluated, and it was found that patients with a tMg of < 1.7 or > 2.3 had a higher all-cause mortality than those with a normal tMg on admission. Patients with normal tMg had fewer cardiac arrhythmias and fewer days on the ventilator.[11] In an analysis of over 3500 patients enrolled in the Dallas Heart Study, hypomagnesemia (tMg) was found to be an independent predictor of mortality: for every 0.2 mg/dL drop in tMg, there was a 20-40% increase in all-cause mortality in patients with and without chronic kidney disease (CKD) (Figure 5).[12]

Figure 5: All-cause mortality related to tMg in patients with and without CKD [12] SMg, serum magnesium
A large meta-analysis also found an inverse correlation between tMg levels and cardiovascular disease, with a 30%decrease in risk for every 0.2 mmol/L rise in tMg.[13] The Atherosclerosis Risk in Communities study, a large population-based study, found a link between low tMg and CKD, with lower tMg levels predicting a higher risk of CKDand ESRD (Figure 6).[14]

Figure 6: Risk of CKD (top) and ESRD (bottom) based on tMg quartile [14]

The exact mechanisms by which hypomagnesemia causes increased morbidity and mortality remain to be fully elucidated, but many sound theories have been proposed. Given the nature of Mg’s role in multiple physiologic processes, there are likely several pathways, and they can be broken down into acute and chronic effects. In terms of the acute effects, cardiac arrhythmias due to an altered electrochemical environment likely play a big role in sudden death.[15] An additional acute negative event relates to hypomagnesemia in patients with acute infections or sepsis. It has been observed that ICU patients with sepsis and low iMg or tMg have a higher mortality.[10, 16] This could be due to a more aggressive cytokine storm, as Mg is known to play a role in attenuating cytokine production, particularly Il-1, Il-6, and TNF.[1, 10, 12, 17, 18] The common thread of the chronic effects is hypomagnesemia’s role in promoting atherosclerotic changes, which can affect numerous organ systems. Low Mg can accelerate the development of atherosclerosis by several avenues:
- Endothelial cell dysfunction. It is known that low Mg has an inhibitory effect on endothelial cell proliferation, which can lead to thrombotic or inflammatory changes in arteries.[19]
- Vascular smooth-muscle calcification. Mg deficiency promotes formation of hydroxyapatite crystals, which can lead to calcification of smooth muscle in arteries, a hallmark of atherosclerosis.[20, 21] It may also mediate calcification by its effect on membrane transport of calcium.[22]
- Hypercoagulability. Magnesium is known to inhibit platelet aggregation, thus low Mg may lead to a thrombotic state.[23]
- Increased inflammation. It is well-known that inflammation plays a role in the development and progression of atherosclerotic plaques.[24]
These findings are reinforced by a study done in hemodialysispatients in Japan.[25] In over 140,000 dialysis patients, both highand low Mg were predictors of mortality (Figure 7). Althoughelevated Mg was not an independent predictor, low Mg was a significant and concentration-dependent predictor of mortality from cardiovascular disease and mortality due to infection.[25] “The major finding of our study is that a lower serum Mg level was a significant and independent predictor of CVD mortalityamong chronic hemodialysis patients. We also found a significant association between hypomagnesemia and non-CVD mortality, especially deaths from infection.”[25]

Figure 7: All-cause mortality related to tMg in a cohort of dialysis patients [25]
Although these data all show correlation, they do not necessarily prove causation. It is possible that low Mg is an epiphenomenon, or a consequence of the illness, but based on what we know about the physiology of Mg it seems likely that it is at least partially responsible. Several studies suggest this to be the case. In the Framingham Heart Study cohort, patients with a higher dietary intake of Mg had significantly lower rates of coronary artery calcification and aortic calcification.[26] In dialysis patients, using a high-Mg dialysate keeps iMg levels in the normal to slightly elevated range. [27, 28] Hypomagnesemia can be a problem for dialysis patients because foods rich in Mg are also generally rich in potassium. Recently, an effort has been made to keep Mg on the high-normal side in dialysis patients by using high-Mg dialysate. A preliminary study evaluating a cohort of these patients in Germany showed that patients in the high-Mgdialysate group had a significantly higher iMg, and had a significantly lower all-cause and cardiovascular 3-year mortality (14.5% vs 0% after adjusting for age and comorbidities).[28] (Figure 8) These studies, and similar ones [29] suggest that maintaining a Mg level on the high-normal to slightly elevated side is beneficial for overall health.

Figure 8: Mortality by Mg dialysate concentration [28]
Summary
- Magnesium is an important electrolyte which plays a key role in numerous physiologic activities.
- Most of the body’s magnesium resides in muscle and bone, with only about 1% in the extracellular space.
- This 1% of extracellular magnesium is critically important for maintaining homeostasis in many critical systems including ion transport, inflammation, muscle function, cardiac function, cell signaling, neurologic function, and coagulation.
- The physiologically active form of magnesium is the ionized fraction, which can vary widely from person to person and generally represents 55-70% of the extracellular magnesium.
- Most analyzers measure total magnesium rather than ionized magnesium, but total magnesium does not accurately represent ionized magnesium in roughly one-third of individuals.
- There is no accurate formula for converting total magnesium to ionized magnesium thus it is critically important to measure ionized magnesium to assess magnesium status, especially in critically ill patients, patients at the extremes of age, perioperative patients, and patients with kidney disease.
- Both chronically high and low magnesium can increase all-cause mortality. Acutely low magnesium can cause sudden cardiac death and potentially result in a more severe inflammatory response to infection.
- Chronically low magnesium seems to paly a role in accelerating atherosclerosis.
- Magnesium supplementation may help mitigate some of its negative effects.
Bibliography
1. Baaij, J.H.F.d., J.G.J. Hoenderop, and R.J.M. Bindels, Magnesium in Man: Implications for Health and Disease. Physiological Reviews, 2015. 95(1): p. 1-46.
2. Saha, H., et al., Serum ionized versus total magnesium in patients with chronic renal disease. Nephron, 1998. 80(2): p. 149-52.
3. Johansson, M. and P.A. Whiss, Weak relationship between ionized and total magnesium in serum of patients requiring magnesium status. Biol Trace Elem Res, 2007. 115(1): p. 13-21.
4. Yeh, D.D., et al., Total and ionized magnesium testing in the surgical intensive care unit - Opportunities for improved laboratory and pharmacy utilization. J Crit Care, 2017. 42: p. 147-151.
5. Huijgen, H.J., et al., Magnesium Levels in Critically Ill Patients: What Should We Measure? American Journal of Clinical Pathology, 2000. 114(5): p. 688-695.
6. Zhan, J., et al., Circulating Ionized Magnesium as a Measure of Supplement Bioavailability: Results from a Pilot Study for Randomized Clinical Trial. Nutrients, 2020. 12(5): p. 1245.
7. Cunningham, J., M. Rodríguez, and P. Messa, Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clinical Kidney Journal, 2012. 5(Suppl_1): p. i39-i51.
8. Elliott, J.P., J.C. Morrison, and J.A. Bofill, Risks and Benefits of Magnesium Sulfate Tocolysis in Preterm Labor (PTL). AIMS public health, 2016. 3(2): p. 348-356.
9. Kreepala, C., et al., Assessment of preeclampsia risk by use of serum ionized magnesium-based equation. Renal failure, 2018. 40(1): p. 99-106.
10. Escuela, M.P., et al., Total and ionized serum magnesium in critically ill patients. Intensive Care Med, 2005. 31(1): p. 151-6.
11. Cheungpasitporn, W., C. Thongprayoon, and Q. Qian, Dysmagnesemia in Hospitalized Patients: Prevalence and Prognostic Importance. Mayo Clin Proc, 2015. 90(8): p. 1001-10.
12. Ferrè, S., et al., Association of serum magnesium with all-cause mortality in patients with and without chronic kidney disease in the Dallas Heart Study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 2018. 33(8): p. 1389-1396.
13. Del Gobbo, L.C., et al., Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta- analysis of prospective studies. The American Journal of Clinical Nutrition, 2013. 98(1): p. 160-173.
14. Tin, A., et al., Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney International, 2015. 87(4): p. 820-827.
15. DiNicolantonio, J.J., J. Liu, and J.H. O’Keefe, Magnesium for the prevention and treatment of cardiovascular disease. Open heart, 2018. 5(2): p. e000775-e000775.
16. Soliman, H.M., et al., Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med, 2003. 31(4): p. 1082-7.
17. de Baaij, J.H.F., J.G.J. Hoenderop, and R.J.M. Bindels, Regulation of magnesium balance: lessons learned from human genetic disease. Clinical kidney journal, 2012. 5(Suppl 1): p. i15-i24.
18. Velissaris, D., et al., Hypomagnesemia in Critically Ill Sepsis Patients. Journal of clinical medicine research, 2015. 7(12): p. 911-918.
19. Maier, J.A., et al., Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta, 2004. 1689(1): p. 13-21.
20. Kircelli, F., et al., Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant, 2012. 27(2): p. 514-21.
21. van de Wal-Visscher, E.R., J.P. Kooman, and F.M. van der Sande, Magnesium in Chronic Kidney Disease: Should We Care? Blood purification, 2018. 45(1-3): p. 173-178.
22. Montezano, A.C., et al., Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension, 2010. 56(3): p. 453-62.
23. Ravn, H.B., et al., Magnesium inhibits human platelets. Blood Coagul Fibrinolysis, 1996. 7(2): p. 241-4.
24. Libby, P., Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology, 2012. 32(9): p. 2045-2051.
25. Sakaguchi, Y., et al., Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int, 2014. 85(1): p. 174-81.
26. Hruby, A., et al., Magnesium intake is inversely associated with coronary artery calcification: the Framingham Heart Study. JACC. Cardiovascular imaging, 2014. 7(1): p. 59-69.
27. Lacson, E., Jr., et al., Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am J Kidney Dis, 2015. 66(6): p. 1056-66.
28. Schmaderer, C., et al., Reduced Mortality in Maintenance Haemodialysis Patients on High versus Low Dialysate Magnesium: A Pilot Study. Nutrients, 2017. 9(9).
29. Leenders, N.H.J. and M.G. Vervloet, Magnesium: A Magic Bullet for Cardiovascular Disease in Chronic Kidney Disease? Nutrients, 2019. 11(2): p. 455.