Original Investigation Caring for the Critically Ill PatientSeptember 2, 2020
Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis
The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group
JAMA. Published online September 2, 2020. doi:10.1001/jama.2020.17023
Abstract 摘要
Importance 背景
Effective therapies for patients with coronavirus disease 2019 (COVID-19) are needed, and clinical trial data have demonstrated that low-dose dexamethasone reduced mortality in hospitalized patients with COVID-19 who required respiratory support.
新冠病毒感染(COVID-19)患者需要有效治疗,临床试验数据显示,小剂量地塞米松能够降低需要呼吸支持治疗的COVID-19住院患者的病死率。
Objective 目的
To estimate the association between administration of corticosteroids compared with usual care or placebo and 28-day all-cause mortality.
评价使用皮质激素和常规治疗或安慰剂与28天全因病死率的相关性。
Design, Setting, and Participants 设计,场景与研究对象
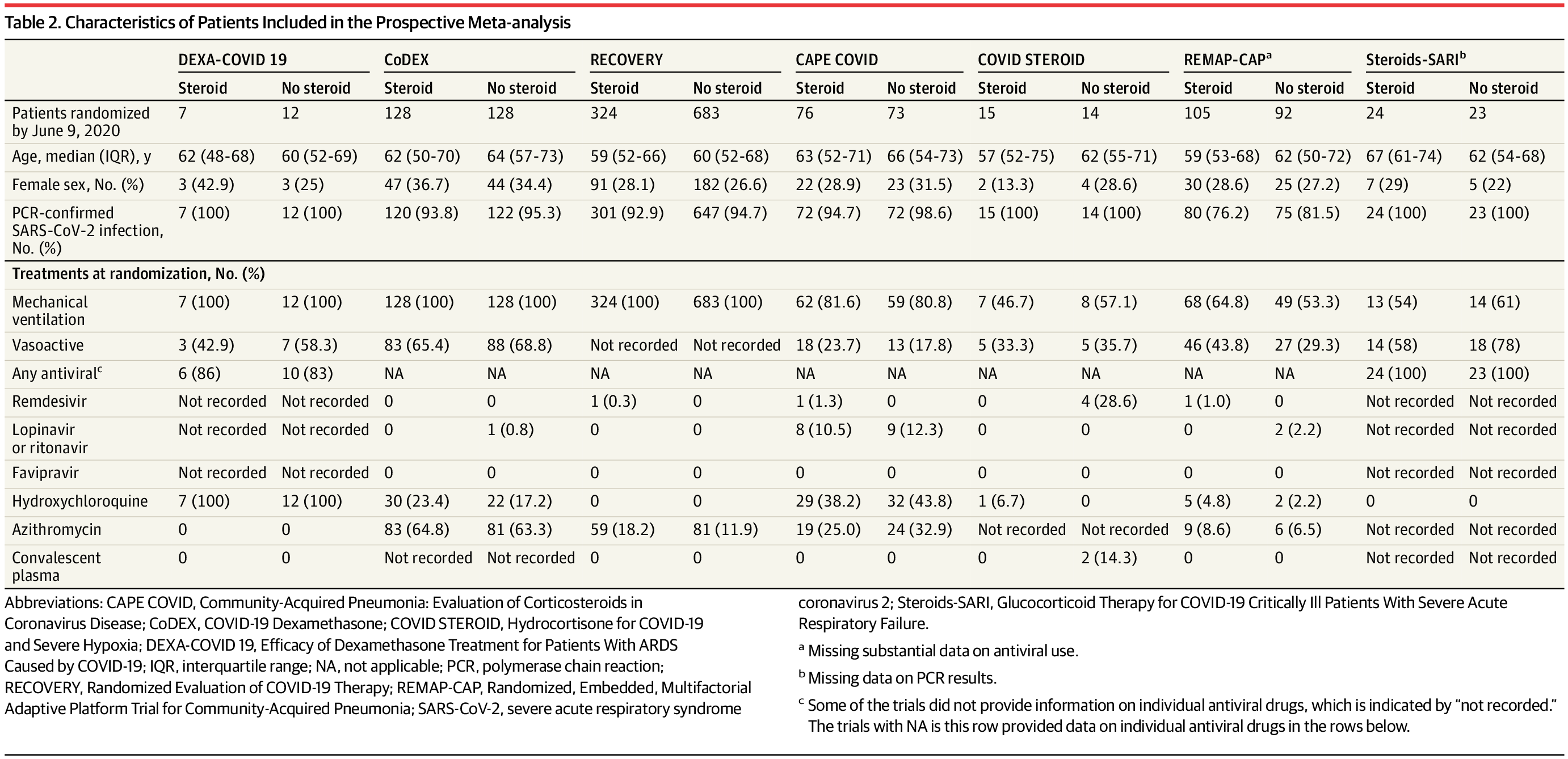
Prospective meta-analysis that pooled data from 7 randomized clinical trials that evaluated the efficacy of corticosteroids in 1703 critically ill patients with COVID-19. The trials were conducted in 12 countries from February 26, 2020, to June 9, 2020, and the date of final follow-up was July 6, 2020. Pooled data were aggregated from the individual trials, overall, and in predefined subgroups. Risk of bias was assessed using the Cochrane Risk of Bias Assessment Tool. Inconsistency among trial results was assessed using the I2 statistic. The primary analysis was an inverse variance–weighted fixed-effect meta-analysis of overall mortality, with the association between the intervention and mortality quantified using odds ratios (ORs). Random-effects meta-analyses also were conducted (with the Paule-Mandel estimate of heterogeneity and the Hartung-Knapp adjustment) and an inverse variance–weighted fixed-effect analysis using risk ratios.
对于7项评价皮质激素疗效的随机对照临床试验的数据进行的前瞻性meta分析,纳入1703名COVID-19危重患者。试验从2020年2月26日至6月9日在12个国家进行,随后随访日期为2020年7月6日。从每项研究收集数据并进行综合分析,且预先确定亚组分析。采用Cochrane偏倚风险评估工具评估偏倚的风险。采用I2 统计变量确定试验结果之间的不一致性。主要分析为针对病死率的逆方差加权固定效应meta分析,采用比数比(OR)定量反映干预措施与病死率的相关性。进行随机效应meta分析(Paule-Mandel异质性评价,Hartung-Knapp校正)和逆方差加权固定效应分析。
Exposures 暴露因素
Patients had been randomized to receive systemic dexamethasone, hydrocortisone, or methylprednisolone (678 patients) or to receive usual care or placebo (1025 patients).
患者接受随机分组,使用全身地塞米松、氢化可的松或甲基强的松龙(678名患者)或使用常规治疗或安慰剂(1025名患者)。
Main Outcomes and Measures 主要预后指标
The primary outcome measure was all-cause mortality at 28 days after randomization. A secondary outcome was investigator-defined serious adverse events.
主要预后指标为随机分组后28天全因病死率。次要预后指标为研究者定义的严重不良事件。
Results 结果
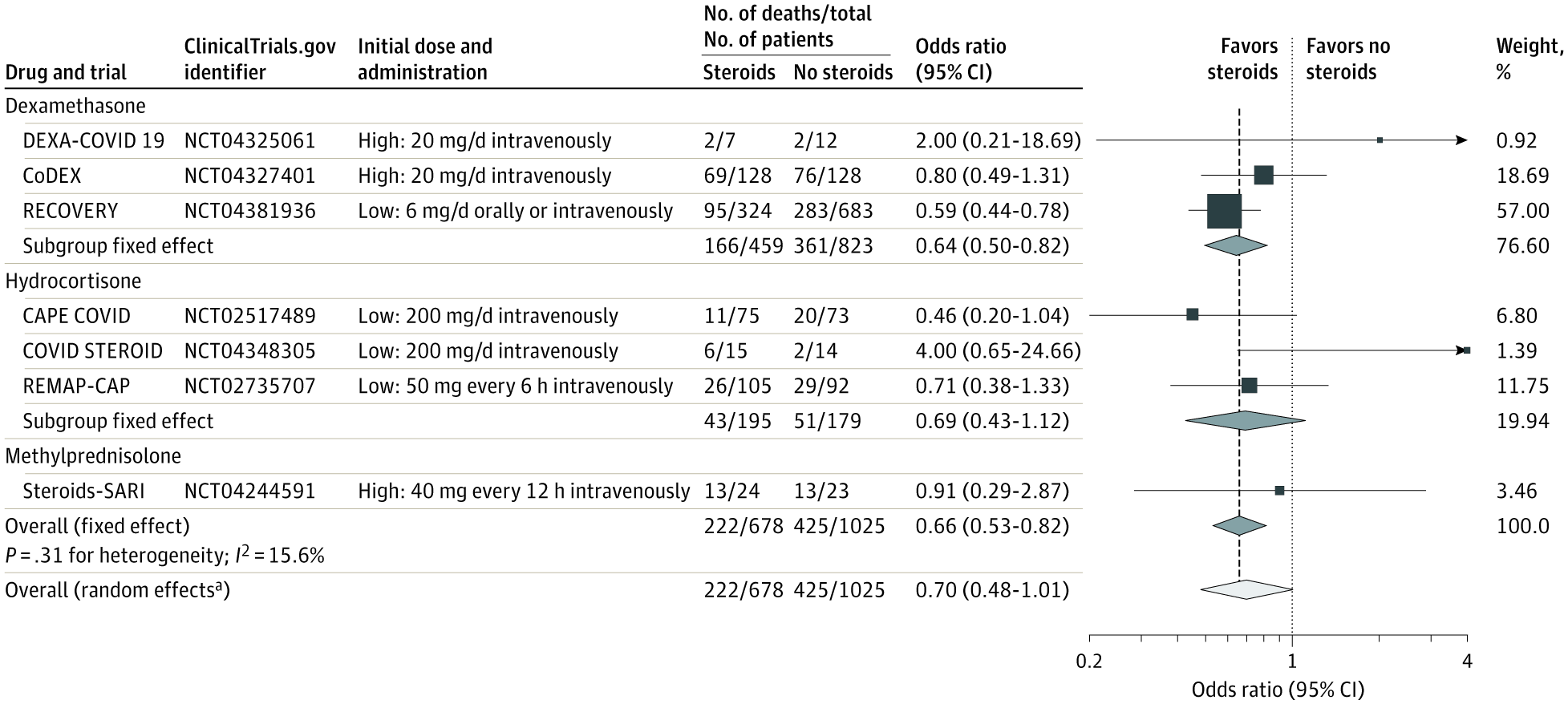
A total of 1703 patients (median age, 60 years [interquartile range, 52-68 years]; 488 [29%] women) were included in the analysis. Risk of bias was assessed as “low” for 6 of the 7 mortality results and as “some concerns” in 1 trial because of the randomization method. Five trials reported mortality at 28 days, 1 trial at 21 days, and 1 trial at 30 days. There were 222 deaths among the 678 patients randomized to corticosteroids and 425 deaths among the 1025 patients randomized to usual care or placebo (summary OR, 0.66 [95% CI, 0.53-0.82]; P < .001 based on a fixed-effect meta-analysis). There was little inconsistency between the trial results (I2 = 15.6%; P = .31 for heterogeneity) and the summary OR was 0.70 (95% CI, 0.48-1.01; P = .053) based on the random-effects meta-analysis. The fixed-effect summary OR for the association with mortality was 0.64 (95% CI, 0.50-0.82; P < .001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths), the OR was 0.69 (95% CI, 0.43-1.12; P = .13) for hydrocortisone (3 trials, 374 patients, and 94 deaths), and the OR was 0.91 (95% CI, 0.29-2.87; P = .87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Among the 6 trials that reported serious adverse events, 64 events occurred among 354 patients randomized to corticosteroids and 80 events occurred among 342 patients randomized to usual care or placebo.
共有1703名患者(中位年龄, 60 岁 [四分位区间, 52-68 岁]; 488 名 [29%] 女性) 纳入分析。7项病死率结果中的6项偏倚风险较低,另外1项研究因随机方法的原因被评估为有些问题。5项研究报告了28天病死率,1项研究报告21天病死率,1项研究报告30天病死率。随机分至皮质激素组的678名患者中222名患者死亡,随机分至常规治疗或安慰剂组的1025名患者中425名患者死亡(基于固定效应meta分析OR, 0.66 [95% CI, 0.53-0.82]; P < .001)。试验结果之间存在轻度不一致(I2 = 15.6%; 异质性P = .31),基于随机效应meta分析OR 为 0.70 (95% CI, 0.48-1.01; P = .053)。与常规治疗或安慰剂相比,地塞米松与病死率相关性的固定效应OR为 0.64 (95% CI, 0.50-0.82; P < .001) (3项试验, 1282名患者, 527名死亡),氢化可的松的OR为 0.69 (95% CI, 0.43-1.12; P = .13) (3项试验, 374名患者, 94名死亡),甲基强的松龙的OR为0.91 (95% CI, 0.29-2.87; P = .87) (1项试验, 47名患者, 26名死亡)。6项试验报告了严重不良事件,皮质激素组354名患者发生64次不良事件,常规治疗或安慰剂组342名患者发生80次不良事件。




Conclusions and Relevance 结论和意义
In this prospective meta-analysis of clinical trials of critically ill patients with COVID-19, administration of systemic corticosteroids, compared with usual care or placebo, was associated with lower 28-day all-cause mortality.
这项针对COVID-19危重患者临床试验的前瞻meta分析显示,与常规治疗或安慰剂相比,全身使用皮质激素伴随28天全因病死率降低。