ICU Management & Practice, Volume 17 - Issue 3, 2017
Innovations in monitoring: from smartphones to wearables
There are over 320 million inpatient surgical procedures worldwide (Weiser et al. 2015). We learnt from the recent International Surgical Outcomes (ISOS) Study (ISOS Group 2016) that around 17% of these patients develop one or more complications, and among them, that 2.8% die from their complications. One can therefore estimate that around 1.5 million patients/yr (4500 patients/ day or 3 patients/min) die of postoperative complications. In the USA, if postoperative mortality was part of the official statistics from the Centers for Disease Control and Prevention, it would represent the third leading cause of death, right after cancer (Bartels et al. 2013). Postoperative complications are not only a human burden. They also dramatically increase hospital costs (Michard et al. 2015).
Facing this clinical and economic burden, several initiatives have been developed to improve quality of surgical care, from the Surgical Safety Checklist, to minimally-invasive surgery, protective mechanical ventilation, and enhanced recovery programmes. Thanks to technological innovations, closer, better and easier monitoring of patients undergoing surgery may also help to improve outcome. Recent advances and perspectives in cardiorespiratory monitoring will be discussed here, from prehabilitation to rehabilitation.
Prehabilitation
Prehabilitation is known to have an impact on postoperative outcome. Preoperative change in physical status and a better control of risk factors can be facilitated by digital tools and applications (apps) downloaded on smartphones or tablets. Connected devices such as wireless brachial cuffs and electronic scales can be used for self-monitoring of blood pressure and weight, the visualisation of trends over time, and a better control of hypertension and overweight before surgery (Michard et al. 2017a). Digital games have been developed for smoking cessation, and multiple activity trackers and apps now exist to invite patients to monitor and increase their physical activity. These digital tools seem to be effective only for a short period of time, which is clearly an issue when dealing with chronic conditions and diseases, but less of a problem if used during the few weeks preceding surgery (Michard et al. 2017a).
Intraoperative management
Fluid management
Intraoperative fluid management is a key determinant of postoperative outcome. Fluid overload has been known for a while as responsible for complications related to tissue oedema (e.g. anastomotic leak, prolonged mechanical ventilation), so that fluid restriction has been encouraged at some point. However, recent studies have clearly demonstrated that insufficient fluid administration is also associated with a significant increase in postoperative complications (Thacker et al. 2016). Therefore titrating or tailoring fluid administration to individual needs is highly desirable to ensure patients receive the right amount of fluid at the right time. Multiple noninvasive haemodynamic monitoring solutions are now available, from bioimpedance tracheal tubes, to bioreactance surface electrodes, applanation tonometry and volume clamp methods (Michard et al 2017a). They give clinicians the opportunity to measure and track changes in blood flow during therapeutic interventions and to rationalise fluid administration. Preventing unjustified fluid administration by detecting fluid unresponsiveness has been shown to be useful to decrease postoperative morbidity, hospital length of stay and costs (Benes et al. 2014; Michard et al. 2017b). Noninvasive parameters such as the pleth variability index (PVI) from pulse oximeters and pulse pressure variation (PPV) from volume clamp methods are useful to detect fluid unresponsiveness.
Blood pressure management
A strong relationship has been established between intraoperative hypotension and postoperative complications, such as stroke, myocardial and acute kidney injury. Intermittent blood pressure measurements do not allow capture of all hypotensive events in a timely manner. Studies suggest that we may miss around 7 minutes of hypotension per hour during typical 3-hour abdominal and orthopaedic procedures when using intermittent measures of blood pressure from a brachial cuff (Chen et al. 2012). It has recently been suggested that only a few minutes of hypotension are susceptible to affect postoperative outcome (Salmasi et al. 2017). Therefore, although causality between intraoperative hypotension and postoperative complications has not yet been established, it seems reasonable to avoid hypotension as much as we can. This may require a more rational and controlled use of anaesthetic agents, in particular during induction, as well as the continuous monitoring of blood pressure with noninvasive techniques for the immediate detection and correction of any significant blood pressure drop.
Blood management
An app has been developed to quantify blood loss in surgical sponges just by taking a picture of them. First studies suggest it works better than methods currently used for surgical blood loss estimation (Konig et al. 2017). Although measuring absolute values of haemoglobin noninvasively remains a challenge, tracking changes over time is now doable with an accept- able level of accuracy (Marques et al. 2015). These new methods may help to rationalise blood transfusion and decrease associated complications.
Postoperative management
Recent studies have shown that around one-third of deaths after surgery occur in the wards (ISOS Group 2016). This situation has been called “failure to rescue”, and has been proposed to explain why hospitals with comparable postoperative morbidity rates may have very different mortality rates: outcome depends more on the ability to detect early and treat properly postoperative complications than in the occurence of complications (Ghaferi et al. 2009). Importantly, many studies have shown that most ward patients start deteriorating hours before medical teams are called for rescue or intensive care unit (ICU) transfer. In this regard, several studies have already demonstrated the value of early detection with continuous monitoring systems.
The use of pulse oximeters to continuously monitor SpO2 and heart rate in 2,841 orthopaedic patients (many of them receiving opioids) was associated with a significant decrease in the number of rescue events and ICU transfers (Taenzer et al. 2010). The use of a piezoelectric contact-free sensor (placed under the mattress) to continuously monitor heart rate and respiratory rate in 2,314 medico-surgical patients was associated with a significant decrease in the number of calls for cardiac arrest and hospital length of stay (Brown et al. 2014). More recently, the use of wireless sensors to monitor vital signs (SpO2, heart rate, blood pressure, respiratory rate), automatically calculate an early warning score and alert nurses in case of deterioration, was associated with a significant decrease in the number of cardiac arrests and in mortality (Subbe et al. 2017).
Multiple sensors and monitoring systems are now available for proactive monitoring in ambulatory patients (Michard et al. 2017a). Smart software has been developed to filter artifacts and prevent alarm fatigue, to fuse vital signs into wellness indexes or warning scores, which are used for the easy and visual detection of clinical deterioration, or even the prediction of adverse events beforehand (Michard et al. 2017c; Pinsky et al. 2016).
Rehabilitation
Activity trackers
Early mobilisation is a key element of postoperative recovery. Multiple wrist, waist or ankle sensors with accelerometers and gyroscopes are available to detect movement or body posture, and hence quantify physical activity. Some have been used with success for the objective assessment of early mobilisation. However, some are not sensitive enough to be used during low-speed exercises, which is often the case immediately after surgery (Michard et al. 2017a).
Electronic checklists
These are apps developed to optimise communication between patients and healthcare professionals during the entire surgical journey. Before surgery they can be used to ensure patients follow preoperative recommendations, in particular regarding medications. After surgery, and once patients have been discharged from the hospital, they can help to detect and describe complications (e.g., wound pictures can be shared) and provide guidance to patients.
Economic impact of technological innovations
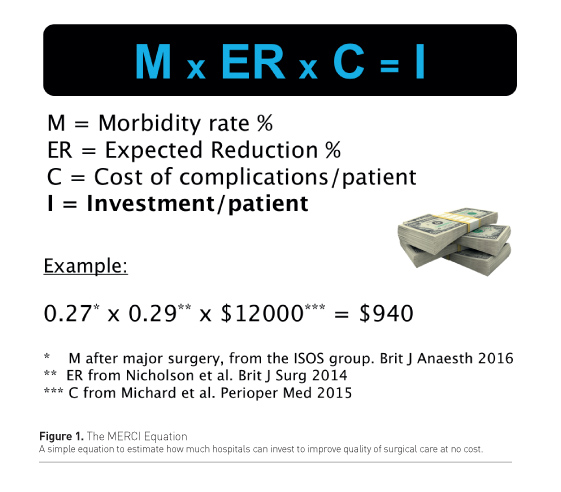
The use and implementation of any new technology has a cost that must be balanced with potential savings associated with the expected improvement in postoperative recovery. Postoperative complications are expensive to treat, and prolong hospital length of stay (increase in hospital cost), reducing opportunities to free beds for new surgeries (decrease in hospital revenues). The MERCI equation has been proposed to simply and quickly estimate how much hospitals could invest to improve quality of surgical care at no cost (Michard 2016a). In this equation, M is the morbidity rate (or proportion of patients who develop at least one postoperative complication), ER is the expected reduction in postoperative morbidity with the new strategy to be implemented, C is the cost difference between patients with and without complications. Then, I gives an estimate of the possible investment to decrease postoperative morbidity at no cost (Figure 1). According to a recent systematic review and meta-analysis, enhanced recovery programmes are capable of decreasing postoperative complications by around 29% (Nicholson et al. 2014). The large International Surgical Outcomes Study (ISOS Group 2016) suggests that the average postoperative morbidity rate of patients undergoing major surgery is 27%, and we know from another recent study (Michard et al. 2015) that the average cost of complications per patient is around US$12,000. In this major surgery context, the MERCI equation shows that the maximum investment per patient (to improve quality of care at no cost) is around $940 (Figure 1). This is a significant amount of money, but it will not allow the purchase and implementation of all technologies described in this article. When investing in new monitoring equipment, assuming an outcome benefit has been demonstrated or is suspected, the MERCI equation helps to build a business case and define priorities.
Looking forward: all in one
Thanks to computer science and bioengineering innovations, monitoring systems will continue to evolve quickly. Stretchable and flexible electronics enable the measurement of blood pressure with tiny and inexpensive sensors made of materials “feeling” our pulse (Michard 2016b). When positioned on radial and carotid arteries, these sensors record and transmit wirelessly high-quality blood pressure curves to any display, from a large screen to a watch. If used in combination with pulse contour algorithms, they will open the door to the remote and ambulatory monitoring of advanced haemodynamic parameters (Michard 2016b). Other variables, such as arterial oxygen saturation, respiratory rate, haemoglobin, temperature and activity can already be monitored noninvasively and continuously. Therefore, it is only a matter of time before all these physiologic variables are integrated into the same sensor or monitoring system (Figure 2).
Conclusion
Postoperative complications are a major clinical and economic burden. Each year, they are responsible for over 1 million deaths. Many complications and deaths could be prevented with better pre- and intraoperative management, the earlier detection of adverse events and more informed therapeutic decisions. The digital revolution is transforming medicine, and physiologic monitoring should dramatically benefit from ongoing hardware and software innovations. New and future monitoring tools have the potential to help us improve quality of surgical care from prehabilitation to rehabilitation. In particular, wireless and wearable sensors can help to detect clinical deterioration at a very early stage in ward patients. By triggering timely interventions, they have the potential to decrease the number of ICU admissions, cardiac arrests and postoperative deaths. The next chapter of physiologic monitoring might be written beyond the operating room and the ICU.