Research
Non-invasive high frequency oscillatory ventilation for primary respiratory support in extremely preterm infants: multicentre randomised controlled trial
Yang Li, Xingwang Zhu, Ling-Jun Li, et al
BMJ 2025; 391 doi: https://doi.org/10.1136/bmj-2025-085569 (Published 06 October 2025)Cite this as: BMJ 2025;391:e085569
Abstract
Objective To test the hypothesis that non-invasive high frequency oscillatory ventilation (NHFOV) is more efficacious than nasal continuous positive airway pressure (NCPAP) in reducing invasive mechanical ventilation as primary respiratory support for extremely preterm infants with respiratory distress syndrome.
Design A multicentre, randomised controlled trial.
Setting Twenty tertiary neonatal intensive care units in China.
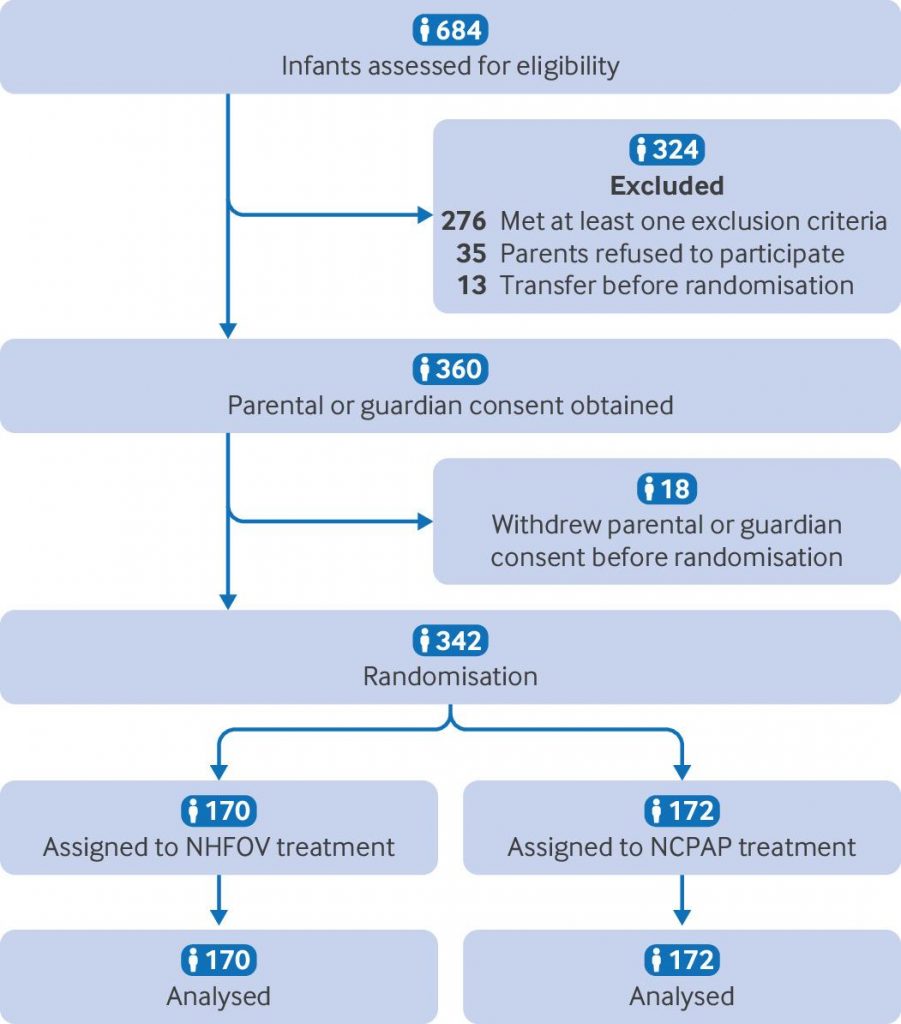
Participants 342 extremely preterm infants (gestational age between 24 weeks +0 day and 28 weeks +6 days) with respiratory distress syndrome were enrolled in the study between August 2022 and August 2024.
Interventions Participants were randomly allocated to receive NCPAP or NHFOV as primary respiratory support for respiratory distress syndrome.
Main outcome measures The primary outcome was treatment failure, defined as the need for invasive mechanical ventilation within 72 hours after birth.
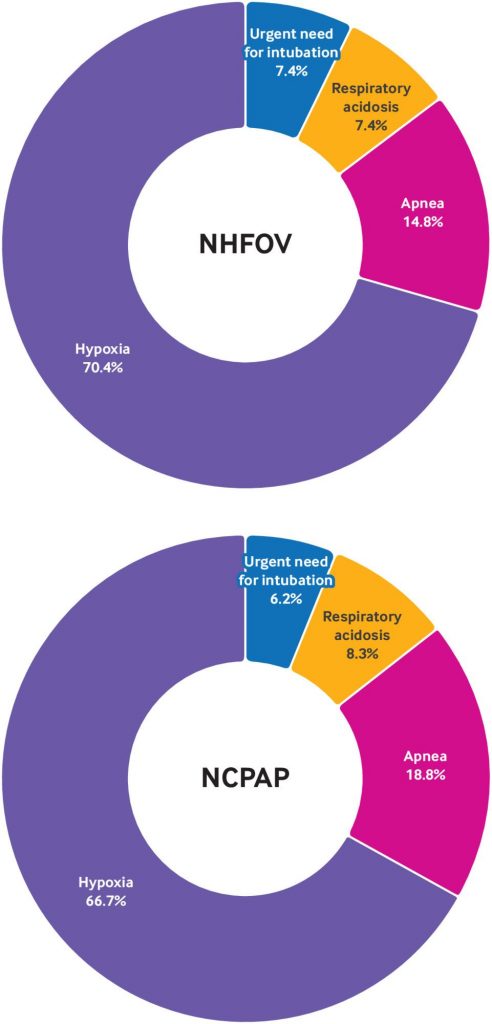
Results Treatment failure within 72 hours occurred in 27 of` 170 infants (15.9%) in the NHFOV group and 48 of 172 infants (27.9%) in the NCPAP group (risk difference −12.0 percentage points, 95% confidence interval −20.7 to −3.4; P=0.007). Treatment failure within seven days was also lower in the NHFOV group (−12.5 percentage points, 95% confidence interval −21.9 to −3.2; P=0.008) compared with the NCPAP group. All observed associations remained significant after sensitivity analysis including study sites and antenatal steroid use. No significant differences were found in any other secondary outcomes between the two groups.
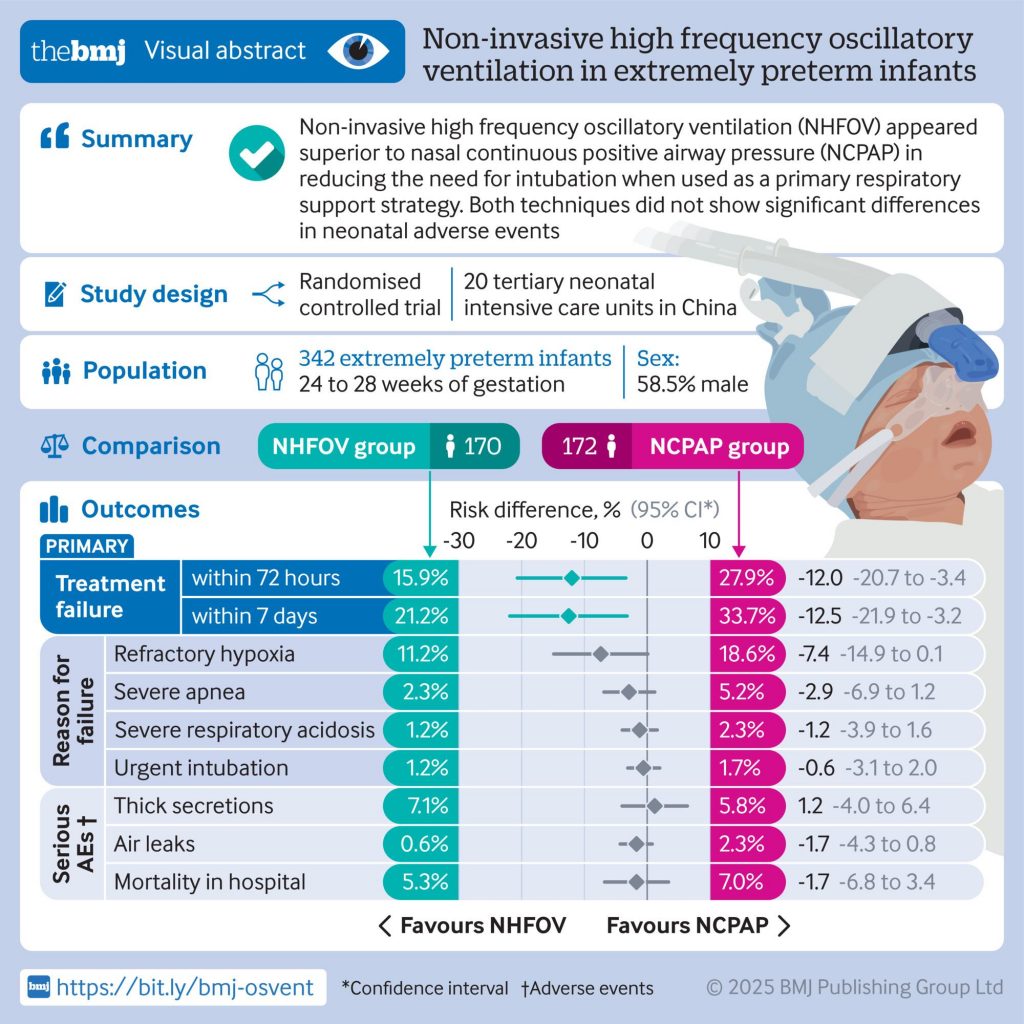
Conclusions NHFOV appeared superior to NCPAP in reducing the need for intubation when used as a primary respiratory support strategy in extremely preterm infants. Both techniques did not show significant differences in neonatal adverse events.
Trial registration ClinicalTrials.gov NCT05141435